AMSTERDAM (dpa-AFX) - Multiple biotech companies are gaining traction as they hit fresh 52-week highs, driven by clinical advancements, regulatory milestones, and strategic funding initiatives. Amylyx Pharmaceuticals, Amneal Pharmaceuticals, and argenx SE each marked notable gains on September 10, 2025, with upcoming catalysts that could further shape their growth trajectories in the months ahead.
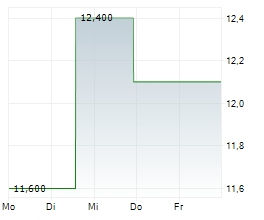
Amylyx Pharmaceuticals Inc. (AMLX), on September 10, 2025, reached a new 52-week high of $12.25, marking a dramatic upswing from its previous low of $2.47. Coinciding with this stock performance, Amylyx announced a $175 million underwritten public offering of common stock.
The offering includes 17.5 million shares priced at $10.00 each, with underwriters granted a 30-day option to purchase up to an additional 2.625 million shares. The proceeds from this offering are expected to support the commercial launch of avexitide, a first-in-class GLP-1 receptor antagonist currently in Phase 3 trials for post-bariatric hypoglycemia and congenital hyperinsulinism. Funds will also be allocated toward ongoing research and development efforts, general corporate purposes, and working capital.
Amylyx's pipeline remains robust despite recent setbacks. The company recently discontinued its ORION program for AMX0035 in progressive supranuclear palsy following disappointing trial results. However, it continues to advance other candidates, including AMX0035 for Wolfram syndrome, AMX0114 for ALS, and a long-acting GLP-1 receptor antagonist for rare metabolic disorders.
**
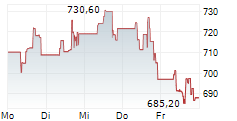
Shares of Amneal Pharmaceuticals Inc. (AMRX), on Sept. 10, showed strong upward momentum, reaching a new 52-week high of $9.97 during intraday trading. The stock opened at $9.82 and traded within a range of $9.77 to $9.97 throughout the day. Despite a modest pullback, AMRX closed at $9.82, reflecting a slight decline of 0.71% from the previous close of $9.89.
The stock's recent performance has been bolstered by a series of positive developments, including the U.S. FDA approval of its risperidone extended-release injectable suspension, a generic version of Risperdal Consta. This approval expands Amneal's central nervous system portfolio and positions the company to capture additional market share in treatments for schizophrenia and bipolar I disorder.
Amneal also has a number of other near-term catalysts. The company is awaiting FDA decisions on its biosimilar candidates for denosumab, a drug used to treat osteoporosis and cancer-related bone loss. Regulatory approval, expected later in 2025, could open up a significant revenue stream. Amneal is also expanding its specialty portfolio, with continued rollout of Brekiya, a DHE autoinjector for migraine and cluster headache, and growing adoption of CREXONT for Parkinson's disease. Another potential catalyst is its strategic collaboration with Metsera to develop GLP-1 therapies for metabolic disorders, which may yield early-stage updates or trial initiations before the end of the year.
We highlighted the stock, AMRX, on April 7, 2025, when it was trading around $7.13. Since then, the stock rose and reached a 52-week high of $9.97 on September 10, representing a gain of 39.8%. Click here to read more on AMRX.
**
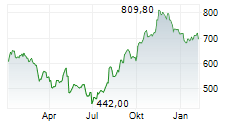
argenx SE (ARGX), a global immunology company focused on treating severe autoimmune diseases, reached a new 52-week high on September 10, 2025. The stock traded as high as $779.03 during the session, setting a fresh annual peak before closing slightly lower at $762.89. This movement reflects a modest intraday decline of 0.54%, down from the previous close of $767.00.
The surge in share price follows a series of bullish developments, including positive topline results from the ADAPT-SERON trial evaluating VYVGART in seronegative generalized myasthenia gravis (gMG). The study met its primary endpoint, demonstrating statistically significant improvements across all gMG subtypes, which positions argenx to pursue FDA label expansion for VYVGART. This could substantially broaden the drug's market reach and revenue potential, especially as VYVGART is already approved for gMG, immune thrombocytopenia (ITP), and chronic inflammatory demyelinating polyneuropathy (CIDP) across multiple regions.
Looking ahead, on September 16, 2025, argenx will host an R&D spotlight webinar focused on ARGX-119, a MuSK agonist being developed for congenital myasthenic syndromes and amyotrophic lateral sclerosis. Additionally, label expansion decisions for VYVGART-SC in Japan and Canada are expected by year-end, alongside topline data readouts for ocular MG and primary ITP in 2026. These milestones could further validate argenx's 'pipeline-in-a-product' strategy and reinforce its leadership in FcRn biology.
***
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News