| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
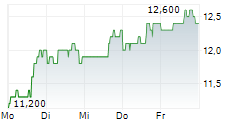
| 11,500 | 11,600 | 11:23 | |
| 11,600 | 11,800 | 27.02. |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Fr | Amneal Pharmaceuticals, Inc. - 10-K, Annual Report | 1 | SEC Filings | ||
| Fr | Amneal outlines 2026 guidance with 12% to 24% EPS growth target, signals acceleration in affordable medicines | 2 | Seeking Alpha | ||
| Fr | Amneal Q4 2025 slides: specialty surge drives 11% revenue growth | 2 | Investing.com | ||
| Fr | Generic Drugs Focused Amneal Pharmaceuticals Revenue Crosses $800 Million | 2 | Benzinga.com | ||
| Fr | Amneal übertrifft Q4-Prognosen, gibt aber verhaltenen Ausblick für 2026 | 1 | Investing.com Deutsch | ||
| Fr | Amneal Pharma A: EPS übertrifft Schätzungen um 0,03 $ - Umsatz besser als erwartet | 1 | Investing.com Deutsch | ||
| AMNEAL PHARMACEUTICALS Aktie jetzt für 0€ handeln | |||||
| Fr | Amneal Pharmaceuticals, Inc. - 8-K, Current Report | - | SEC Filings | ||
| Fr | Amneal Pharmaceuticals Non-GAAP EPS of $0.21 beats by $0.03, revenue of $814M beats by $6.68M | 2 | Seeking Alpha | ||
| Fr | Amneal Pharmaceuticals, Inc.: Amneal Reports Fourth Quarter and Full Year 2025 Financial Results | 83 | GlobeNewswire (Europe) | - Full Year 2025 Performance Met or Exceeded All Guidance Metrics and 2026 Reflects Another Year of Growth - - Q4 2025 Net Revenue of $814 million; GAAP Net Income of $35 million; Diluted Income per... ► Artikel lesen | |
| Fr | Amneal Pharmaceuticals Q4 Earnings Summary & Key Takeaways | 2 | Benzinga.com | ||
| Do | Amneal Pharmaceuticals Q4 2025 Earnings Preview | 2 | Seeking Alpha | ||
| Do | Earnings Outlook For Amneal Pharmaceuticals | 3 | Benzinga.com | ||
| 18.02. | Amneal Pharmaceuticals, Inc. (AMRX) Added to S&P SmallCap 600 Index, Boosting Investor Visibility | 3 | Insider Monkey | ||
| 03.02. | Amneal Pharmaceuticals, Inc. - 8-K, Current Report | - | SEC Filings | ||
| 30.01. | If You Invested $100 In Amneal Pharmaceuticals Stock 5 Years Ago, You Would Have This Much Today | 9 | Benzinga.com | ||
| 29.01. | Amneal initiates nationwide settlement for opioid-related claims | 4 | Seeking Alpha | ||
| 28.01. | Amneal Pharmaceuticals, Inc.: Amneal Pharmaceuticals Added to S&P SmallCap 600 Index | 102 | GlobeNewswire (Europe) | BRIDGEWATER, N.J., Jan. 28, 2026 (GLOBE NEWSWIRE) -- Amneal Pharmaceuticals, Inc. (Nasdaq: AMRX) ("Amneal" or the "Company") today announced that it has been added to the S&P SmallCap 600 Index. The... ► Artikel lesen | |
| 28.01. | TTM Technologies, Dutch Bros to join S&P 400 Index; Amneal, Apellis to be part of S&P SmallCap 600 | 11 | Seeking Alpha | ||
| 27.01. | Amneal Pharmaceuticals, Inc.: Amneal to Report Fourth Quarter and Full Year 2025 Results on February 27, 2026 | 2 | GlobeNewswire (USA) | ||
| 14.01. | Amneal and VantAI unveil fresh branding to kick off 2026 plans | 1 | FiercePharma |
1 2 3 4 Weiter >>
74 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIONTECH | 93,10 | -0,21 % | Aufgrund von angeblichen Patentverletzungen zieht BioNTech gegen Moderna vor Gericht | Die Hersteller von Corona-Impfstoffen haben sich schon seit Jahren in den Haaren. Immer wieder gibt es Vorwürfe über Patentverletzungen. So auch in einem aktuellen Fall, bei dem BioNTech gegen den US-Hersteller... ► Artikel lesen | |
| EVOTEC | 5,880 | +0,38 % | Evotec Aktie: Kann man "diesem Braten wirklich trauen"? | © Foto: Julia Koblitz auf Unsplash (Symbolbild)Neue Aufträge, ein Sparprogramm und frische Analystenkommentare. Die Evotec-Aktie sendet wieder Lebenszeichen. Doch wer die Geschichte dieses Hamburger... ► Artikel lesen | |
| BB BIOTECH | 51,30 | -0,19 % | Bayer Aktie: Alles vorbei? - Airbus, BB Biotech, BioNTech, TUI und Valneva - Börse Frankfurt | Wo ist am Aktienmarkt etwas los, welche Themen interessieren Anleger derzeit besonders? Vor allem für Trader ist es wichtig zu wissen, wo "die Musik spielt" und welche Themen an der Börse aktuell besonders... ► Artikel lesen | |
| MEDIGENE | 0,046 | +14,00 % | Medigene: Zurückziehung - 18.11.2025 | ||
| QIAGEN | 41,960 | +1,38 % | Qiagen: Analysten sehen Wettbewerbssorgen als übertrieben | Nach Gesprächen mit dem Qiagen-Management auf Analysten-Konferenzen in den USA stufen Analysten die Aktie des Diagnostikspezialisten weiterhin positiv ein. Jefferies beließ es bei einer Kaufempfehlung... ► Artikel lesen | |
| MODERNA | 45,160 | -0,41 % | Moderna's combination flu, COVID shot wins over European drug regulators | ||
| PAION | 0,090 | 0,00 % | PAION AG - Schwächesignale im Fokus | ||
| VALNEVA | 4,700 | -0,63 % | Original-Research: Valneva (von First Berlin Equity Research GmbH): Buy | Original-Research: Valneva - von First Berlin Equity Research GmbH
25.02.2026 / 17:11 CET/CEST
Veröffentlichung einer Research, übermittelt durch EQS News - ein Service der EQS Group.
Für den Inhalt... ► Artikel lesen | |
| AMGEN | 328,50 | -0,02 % | Is Amgen Stock Outperforming the Dow? | ||
| EPIGENOMICS | 1,050 | 0,00 % | PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025 | DJ PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR
Epigenomics AG: Vorläufiges Jahresergebnis zum... ► Artikel lesen | |
| NOVAVAX | 8,495 | -0,97 % | FOMO an der Wall Street: Novavax meldet überraschenden Gewinn - Aktie schießt zweistellig hoch | © Foto: picture alliance / Ulrich Baumgarten | Ulrich BaumgartenEin Ergebnis, das niemand erwartet hatte, gepaart mit neuen Big-Pharma-Deals: Novavax sorgt für FOMO an der Wall Street. Wie lange hält... ► Artikel lesen | |
| STRYKER | 327,20 | -0,24 % | Think Surgical announces first TMINI robot cases with Stryker knee implant | ||
| BIOGEN | 162,45 | +0,06 % | WONBIOGEN Co., Ltd: Won BioGen Accelerates Global Market Expansion with Advanced Moist Wound Care Technology | SEOUL, South Korea, Feb. 24, 2026 /PRNewswire/ -- Medical device manufacturer Won BioGen (CEO Kim Won-il) is accelerating its global market expansion based on its advanced moist wound care... ► Artikel lesen | |
| BIOFRONTERA | 2,570 | -4,10 % | PTA-Adhoc: Biofrontera AG: Erhöhung Prognose | DJ PTA-Adhoc: Biofrontera AG: Erhöhung Prognose
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR Schlagwort(e): Sonstiges
Biofrontera AG: Erhöhung Prognose
Leverkusen... ► Artikel lesen | |
| HEIDELBERG PHARMA | 2,990 | -1,97 % | HEIDELBERG PHARMA AG im strukturellen Gleichgewicht |
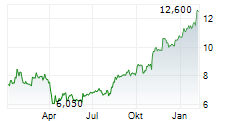
AMNEAL PHARMACEUTICALS INC gehört der Branche Biotechnologie an. Der aktuelle Kurs liegt bei 11,500 Euro und damit 0,00 % im Plus.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu AMNEAL PHARMACEUTICALS INC finden Sie auf Wallstreet Online.