WASHINGTON (dpa-AFX) - Amneal Pharmaceuticals Inc. (AMRX) announced that the U.S. Food and Drug Administration has approved the company's cyclosporine ophthalmic emulsion 0.05%, a sterile, preservative-free formulation supplied in single-use vials. The product is the generic equivalent of RESTASIS (cyclosporine ophthalmic emulsion) 0.05%, a registered trademark of Allergan, an AbbVie company.
Cyclosporine ophthalmic emulsion 0.05% is a topical immunomodulator indicated to increase tear production in patients whose tear production is presumed to be suppressed due to ocular inflammation associated with dry eye syndrome. Increased tear production was not seen in patients currently taking topical anti-inflammatory drugs or using punctal plugs.
The most common adverse reaction associated with cyclosporine ophthalmic emulsion 0.05% was ocular burning.
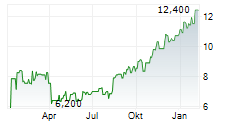
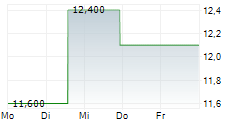
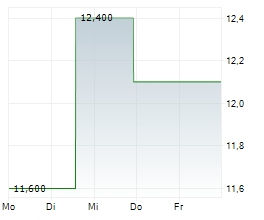
According to IQVIA U.S. annual sales for cyclosporine ophthalmic emulsion 0.05% in sterile, preservative-free single-use vials for the 12 months ended September 2025 were approximately $2.0 billion.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News