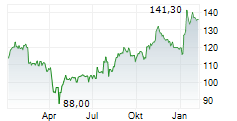
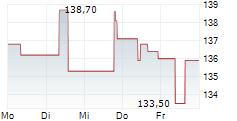
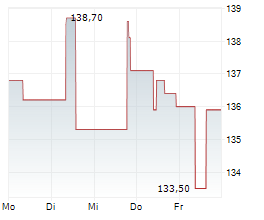
PARIS (dpa-AFX) - Ipsen (IPSEY) announced first aesthetic data for glabellar lines from Stage 1 of its multi-stage, ongoing Phase II LANTIC trial evaluating the internally developed IPN10200. Patients treated with IPN10200 showed a statistically significant improvement in response at Week 4 versus placebo (primary endpoint). A longer duration of effect was observed, with a substantial majority of patients achieving a clinically significant response at Week 24 compared with placebo and Dysport-defined as a score of 'none' or 'mild.' IPN10200 continued to demonstrate superior line severity response versus Dysport at Week 36. Dysport performed consistently with its known clinical profile.
Ipsen announced that data from the Phase II LANTIC trial for IPN10200 will be presented at an upcoming scientific conference in the first half of 2026. Phase III start-up activities have already been initiated. Meanwhile, Phase II development continues for therapeutic indications beyond aesthetics, including adult upper limb spasticity, migraine, and cervical dystonia.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News