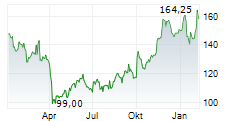
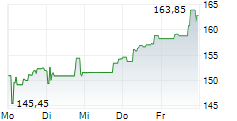
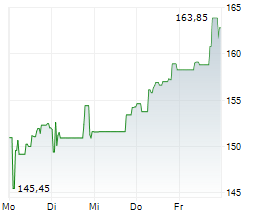
WESTON (dpa-AFX) - Biogen Inc. (Nasdaq: BIIB) announced that the U.S. Food and Drug Administration (FDA) has issued a Complete Response Letter (CRL) for its supplemental New Drug Application or sNDA seeking approval of a higher dose of nusinersen for the treatment of spinal muscular atrophy (SMA).
The FDA did not find any problems with the clinical data. However, it asked Biogen to update the technical details in the Chemistry Manufacturing and Controls (CMC) section of the application.
Biogen said it will use existing information to quickly resubmit the application and address the FDA's request.
The company noted that it is working with regulatory authorities around the world to advance the high dose regimen as an additional dosing option for people living with SMA. The high dose regimen of SPINRAZA (nusinersen) was recently approved in Japan and is actively under review by the European Medicines Agency (EMA) and other global regulators.
SPINRAZA (nusinersen) 12mg/5 mL injection is approved in more than 71 countries to treat infants, children and adults with spinal muscular atrophy (SMA).
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News