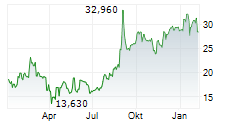
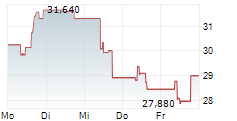
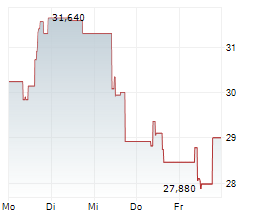
TOKYO (dpa-AFX) - BioArctic AB (BRCTF) announced Wednesday that Australia's Therapeutic Goods Administration (TGA) has approved Leqembi for the treatment of adults with early Alzheimer's disease.
BioArctic holds the rights to commercialize Leqembi, developed in collaboration with Eisai Co Ltd (ESALY.PK), in the Nordic region, and the two companies are preparing for a joint commercialization in the region.
The approval comes after the TGA initially declined Leqembi in February 2025. Eisai requested a review in March, and subsequent discussions with the TGA led to an agreement that secured Leqembi's approval.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News