| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
| 28,880 | 29,120 | 07:05 | |
| 28,700 | 28,940 | 07:05 |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Fr | Number of shares and votes in BioArctic AB (publ) as of February 27, 2026 | 234 | PR Newswire | STOCKHOLM, Feb. 27, 2026 /PRNewswire/ -- BioArctic AB (publ) (Nasdaq Stockholm: BIOA B) announced today that the company issued 78,000 Class B shares during February for delivery of shares... ► Artikel lesen | |
| 20.02. | Biogen und BioArctic: Debatte um Alzheimer-Mittel in Deutschland spitzt sich zu | 430 | Der Aktionär | Im vergangenen Jahr hat Biogen mit seinem japanischen Partner Eisai das Alzheimer-Medikament Leqembi (Lecanemab) auch in Deutschland auf den Markt gebracht. Doch es herrschen Zweifel an der Wirksamkeit... ► Artikel lesen | |
| 19.02. | AKTIONÄR-Hot-Stock BioArctic schießt hoch: Dividenden-Überraschung! | 301 | Der Aktionär | BioArctic, der ursprüngliche Entwickler des Alzheimer-Medikaments Leqembi (Lecanemab), hat am Mittwoch starke Zahlen zum vierten Quartal respektive dem Gesamtjahr vorgelegt. Darüber hinaus sorgt die... ► Artikel lesen | |
| 18.02. | BioArctic Interim Report for the period October - December 2025 | 570 | PR Newswire | STOCKHOLM, Feb. 18, 2026 /PRNewswire/ -- A transformative year with record financial results
Events during the fourth quarter 2025Leqembi Iqlik was launched for weekly maintenance dosing... ► Artikel lesen | |
| BIOARCTIC Aktie jetzt für 0€ handeln | |||||
| 11.02. | Invitation to presentation of BioArctic's fourth quarter report for October - December 2025 on February 18 at 9.30 a.m. CET | 213 | PR Newswire | STOCKHOLM, Feb. 11, 2026 /PRNewswire/ -- BioArctic AB (publ) (NASDAQ Stockholm: BIOA B) will publish the company's fourth quarter report for October - December 2025 on Wednesday, February... ► Artikel lesen | |
| 09.02. | BioArctic: BLA for subcutaneous formulation of Leqembi designated for Priority Review in China | 586 | PR Newswire | STOCKHOLM, Feb. 9, 2026 /PRNewswire/ -- BioArctic AB's (publ) (NASDAQ: BIOA B (Stockholm: BIOA B) partner Eisai announced today that the Biologics License Application (BLA) for the treatment... ► Artikel lesen | |
| 06.02. | BioArctic AB: Sales of Leqembi totaled 20.7 billion yen in the fourth quarter 2025 | 343 | GlobeNewswire (Europe) | Stockholm, Sweden, February 6, 2026 - BioArctic AB's (publ) (Nasdaq Stockholm: BIOA B) partner Eisai today published the preliminary global revenue for Leqembi for the fourth quarter 2025, in conjunction... ► Artikel lesen | |
| 26.01. | BioArctic: Eisai submits Marketing Authorisation Variation to EMA for intravenous maintenance dosing every four weeks with Leqembi (lecanemab) | 465 | PR Newswire | STOCKHOLM, Sweden, Jan. 26, 2026 /PRNewswire/ -- BioArctic AB's (publ) (Nasdaq Stockholm: BIOA B) partner Eisai announced today that they have submitted a proposed Marketing Authorisation... ► Artikel lesen | |
| 26.01. | BioArctic's Partner Eisai Receives Priority Review For Leqembi Iqlik Subcutaneous Autoinjector | 358 | AFX News | TOKYO (dpa-AFX) - BioArctic AB (BRCTF) announced on Monday that its partner Eisai (ESALY) has received Priority Review from the U.S. Food and Drug Administration (FDA) for the supplemental Biologics... ► Artikel lesen | |
| 26.01. | BioArctic: Leqembi Iqlik (lecanemab-irmb) supplemental Biologics License Application regarding subcutaneous starting dose granted Priority Review by the US FDA | 691 | PR Newswire | STOCKHOLM, Jan. 26, 2026 /PRNewswire/ -- BioArctic AB's (publ) (STO: BIOA B) partner Eisai announced today that the supplemental Biologics License Application (sBLA) for Leqembi Iqlik subcutaneous... ► Artikel lesen | |
| 06.01. | BioArctic: BLA for subcutaneous formulation of Leqembi accepted in China | 422 | PR Newswire | STOCKHOLM, Jan. 6, 2026 /PRNewswire/ -- BioArctic AB's (publ) (Nasdaq Stockholm: BIOA B) partner Eisai announced today that the Biologics License Application (BLA) for the subcutaneous formulation... ► Artikel lesen | |
| 09.12.25 | BioArctic: Leqembi included in China's commercial insurance innovative drug list | 464 | PR Newswire | STOCKHOLM, Dec. 8, 2025 /PRNewswire/ -- BioArctic AB's (publ) (Nasdaq Stockholm: BIOA B) partner Eisai announced today that Leqembi (lecanemab), has been included in the "Commercial Insurance... ► Artikel lesen | |
| 04.12.25 | BioArctic: New Leqembi-data presented at CTAD 2025 suggests potential to delay disease progression by up to 8.3 years with continued treatment | 471 | PR Newswire | STOCKHOLM, Dec. 4, 2025 /PRNewswire/ -- BioArctic AB's (publ) (Nasdaq Stockholm: BIOA B) partner Eisai presented the latest findings on time saved with continued treatment with lecanemab... ► Artikel lesen | |
| 02.12.25 | AKTIONÄR-Hot-Stock BioArctic: Debatte um Alzheimer-Medikament - so reagiert die Aktie | 513 | Der Aktionär | Seit dem September ist das Alzheimer-Medikament Leqembi, welches seinen Ursprung in Schweden bei BioArctic hat, auch in Deutschland zugelassen. Ein nun veröffentlichter Report sieht keinen Beweis für... ► Artikel lesen | |
| 19.11.25 | BioArctic: New data on lecanemab to be presented at CTAD conference | 720 | PR Newswire | STOCKHOLM, Nov. 18, 2025 /PRNewswire/ -- BioArctic AB's (publ) (Nasdaq Stockholm: BIOA B) partner Eisai will present the latest findings on lecanemab (Leqembi®) at the Clinical Trials... ► Artikel lesen | |
| 14.11.25 | BioArctic: Leqembi approved for IV maintenance treatment in the United Kingdom | 526 | PR Newswire | STOCKHOLM, Nov. 13, 2025 /PRNewswire/ -- BioArctic AB's (publ) (Nasdaq Stockholm: BIOA B) partner Eisai announced today that Leqembi (lecanemab) has been approved for once every four... ► Artikel lesen | |
| 13.11.25 | Interim Report for the period July - September 2025: BioArctic | 451 | PR Newswire | STOCKHOLM, Nov. 13, 2025 /PRNewswire/ --
Broadening our portfolio with new projects and partnerships
Events during the third quarter 2025
New... ► Artikel lesen | |
| 04.11.25 | Invitation to presentation of BioArctic's third quarter report for July - September 2025 on November 13 at 9.30 a.m. CET | 366 | PR Newswire | STOCKHOLM, Nov. 4, 2025 /PRNewswire/ -- BioArctic AB (publ) (Nasdaq Stockholm: BIOA B) will publish the company's third quarter report for July - September 2025 on Thursday, November... ► Artikel lesen | |
| 30.10.25 | BioArctic: Sales of Leqembi totaled 18 billion yen in the third quarter 2025 | 356 | PR Newswire | STOCKHOLM, Oct. 30, 2025 /PRNewswire/ -- BioArctic AB's (publ) (STO: BIOA B) partner Eisai today published the preliminary global revenue for Leqembi during the third quarter 2025... ► Artikel lesen | |
| 27.10.25 | BioArctic: Health Canada Grants Authorization for Leqembi (lecanemab) | 378 | PR Newswire | STOCKHOLM, Oct. 27, 2025 /PRNewswire/ -- BioArctic AB's (publ) (Nasdaq Stockholm: BIOA B) partner Eisai announced today that Health Canada has issued a Notice of Compliance with Conditions... ► Artikel lesen |
1 2 3 Weiter >>
58 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIONTECH | 93,10 | -0,21 % | Aufgrund von angeblichen Patentverletzungen zieht BioNTech gegen Moderna vor Gericht | Die Hersteller von Corona-Impfstoffen haben sich schon seit Jahren in den Haaren. Immer wieder gibt es Vorwürfe über Patentverletzungen. So auch in einem aktuellen Fall, bei dem BioNTech gegen den US-Hersteller... ► Artikel lesen | |
| EVOTEC | 5,880 | +0,38 % | Evotec Aktie: Kann man "diesem Braten wirklich trauen"? | © Foto: Julia Koblitz auf Unsplash (Symbolbild)Neue Aufträge, ein Sparprogramm und frische Analystenkommentare. Die Evotec-Aktie sendet wieder Lebenszeichen. Doch wer die Geschichte dieses Hamburger... ► Artikel lesen | |
| BB BIOTECH | 51,30 | -0,19 % | BioNTech, Valneva oder BB Biotech: Warum es HIER in Kürze knallt! | Die Biotechbranche steht zu Beginn des Jahres 2026 erneut im Spannungsfeld zwischen wissenschaftlicher Innovation und wachsender Skepsis der Kapitalmärkte. Nach Jahren extremer Kursbewegungen prüfen... ► Artikel lesen | |
| MEDIGENE | 0,046 | +14,00 % | Medigene: Zurückziehung - 18.11.2025 | ||
| QIAGEN | 41,960 | +1,38 % | flatexDEGIRO, Qiagen, Börse München - 4investors-Weekend | Wo ist am Aktienmarkt etwas los, welche Themen interessieren Anleger derzeit besonders? Und welche Titel haben besonders starke Kursbewegungen verzeichnet? Vor allem für Trader ist es wichtig zu wissen... ► Artikel lesen | |
| MODERNA | 45,160 | -0,41 % | Moderna's combination flu, COVID shot wins over European drug regulators | ||
| PAION | 0,090 | 0,00 % | PAION AG - Schwächesignale im Fokus | ||
| VALNEVA | 4,700 | -0,63 % | Original-Research: Valneva (von First Berlin Equity Research GmbH): Buy | Original-Research: Valneva - von First Berlin Equity Research GmbH
25.02.2026 / 17:11 CET/CEST
Veröffentlichung einer Research, übermittelt durch EQS News - ein Service der EQS Group.
Für den Inhalt... ► Artikel lesen | |
| AMGEN | 328,50 | -0,02 % | Is Amgen Stock Outperforming the Dow? | ||
| EPIGENOMICS | 1,050 | 0,00 % | PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025 | DJ PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR
Epigenomics AG: Vorläufiges Jahresergebnis zum... ► Artikel lesen | |
| NOVAVAX | 8,495 | -0,97 % | FOMO an der Wall Street: Novavax meldet überraschenden Gewinn - Aktie schießt zweistellig hoch | © Foto: picture alliance / Ulrich Baumgarten | Ulrich BaumgartenEin Ergebnis, das niemand erwartet hatte, gepaart mit neuen Big-Pharma-Deals: Novavax sorgt für FOMO an der Wall Street. Wie lange hält... ► Artikel lesen | |
| STRYKER | 327,20 | -0,24 % | Think Surgical announces first TMINI robot cases with Stryker knee implant | ||
| BIOGEN | 162,45 | +0,06 % | WONBIOGEN Co., Ltd: Won BioGen Accelerates Global Market Expansion with Advanced Moist Wound Care Technology | SEOUL, South Korea, Feb. 24, 2026 /PRNewswire/ -- Medical device manufacturer Won BioGen (CEO Kim Won-il) is accelerating its global market expansion based on its advanced moist wound care... ► Artikel lesen | |
| BIOFRONTERA | 2,570 | -4,10 % | PTA-Adhoc: Biofrontera AG: Erhöhung Prognose | DJ PTA-Adhoc: Biofrontera AG: Erhöhung Prognose
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR Schlagwort(e): Sonstiges
Biofrontera AG: Erhöhung Prognose
Leverkusen... ► Artikel lesen | |
| HEIDELBERG PHARMA | 2,990 | -1,97 % | HEIDELBERG PHARMA AG im strukturellen Gleichgewicht |
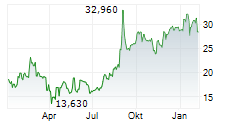
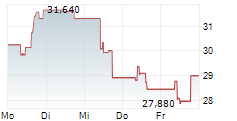
Das Unternehmen BIOARCTIC AB kann der Branche Biotechnologie zugeordnet werden. Die relative Kursveränderung beträgt aktuell +0,21 % bei einem Aktienkurs von 29,280 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu BIOARCTIC AB finden Sie auf Wallstreet Online.