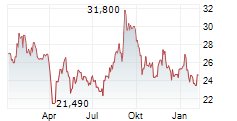
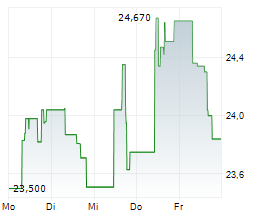
WESTON (dpa-AFX) - Eisai Co., Ltd. (ESALY.PK, ESALF.PK, 4523.T) and Biogen Inc. (BIIB) announced that the National Medical Products Administration (NMPA) of China has approved LEQEMBI-a humanized monoclonal antibody targeting soluble aggregated amyloid-beta (A?)-for intravenous (IV) maintenance dosing once every four weeks.
This approval supports the continued treatment of patients with early-stage Alzheimer's disease, including those with mild cognitive impairment (MCI) or mild dementia. Following an 18-month initiation phase of 10 mg/kg dosing every two weeks, patients may transition to the newly approved maintenance regimen or continue with the biweekly schedule.
In January 2024, LEQEMBI was approved for the treatment of Alzheimer's disease (AD) in patients with mild cognitive impairment (MCI) or mild dementia stage of disease in China.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News