WASHINGTON (dpa-AFX) - Neurocrine Biosciences, Inc. (NBIX), Friday announced a new post-hoc analysis from the Phase 3, open-label KINECT 4 study, evaluating the long-term efficacy, safety and tolerability of INGREZZA in adults with tardive dyskinesia, a movement disorder.
The open-label study found that the patients treated with 40 mg dose of once-daily INGREZZA capsules experienced clinically meaningful improvements in tardive dyskinesia symptoms.
Of the total participants, 90 percent on continuous INGREZZA 40 mg treatment showed 'much improved' or 'very much improved' at Week 48 per clinician assessment and patient self-report.
Moreover, 90 percent of those completing 48 weeks met the Abnormal Involuntary Movement Scale response threshold. Similarly, patients who reduced their dose from 80 mg to 40 mg for tolerability reasons achieved similar therapeutic benefits.
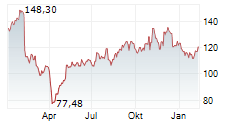
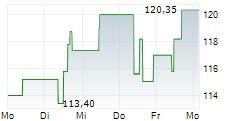
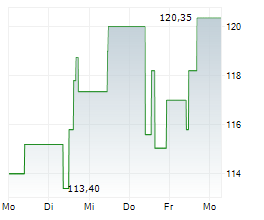
Currently, NBIX is trading at $137.80, up 0.20 percent on the Nasdaq.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News