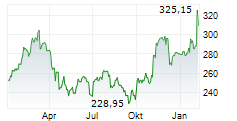
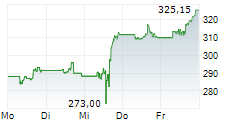
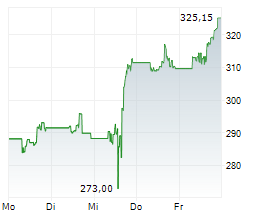
THOUSAND OAKS (dpa-AFX) - AstraZeneca (AZN) and Amgen (AMGN) announced that Tezspire (tezepelumab) has received European Union approval as an add-on therapy with intranasal corticosteroids for adult patients with severe chronic rhinosinusitis with nasal polyps (CRSwNP). The indication targets individuals who have not adequately responded to standard treatments, including systemic corticosteroids and/or surgery.
CRSwNP affects approximately 320 million people worldwide and is a complex disease characterised by epithelial-driven inflammation and benign polyp growths within the nasal cavity. People living with CRSwNP commonly experience airflow obstruction and symptoms including congestion and an impaired sense of smell.
Tezspire was recently approved in the US for the add-on maintenance treatment of adult and paediatric patients aged 12 years and older with inadequately controlled CRSwNP and regulatory applications are currently under review in China, Japan and several other countries. Tezspire is also approved for severe asthma in the US, EU, Japan and more than 60 countries across the globe.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News