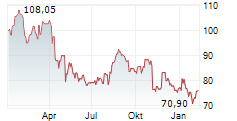
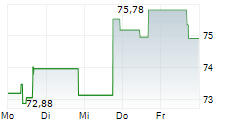
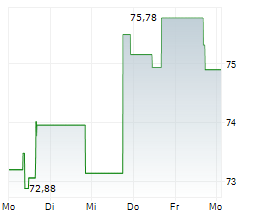
WASHINGTON (dpa-AFX) - Zimmer Biomet Holdings, Inc. (ZBH), a medical technology company, said Tuesday that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Designation for its iodine-treated total hip replacement system.
The iodine technology integrates a controlled-release iodine surface treatment into the company's iTaperloc Complete and iG7 Hip Systems to help address infection risks in joint replacement procedures, particularly for patients at higher risk.
The system was approved by Japan's Pharmaceuticals and Medical Devices Agency (PMDA) in September, making it the world's first approved orthopedic implant with iodine technology.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News