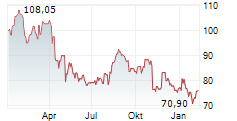
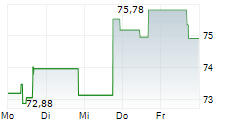
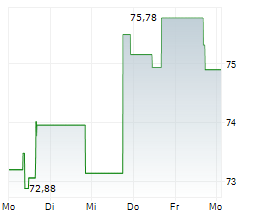
SEOUL (dpa-AFX) - Zimmer Biomet Holdings, Inc. (ZBH) announced FDA 510(k) clearance of ROSA Knee with OptimiZe, an enhanced version of its ROSA Knee System. Zimmer Biomet will conduct a targeted release of ROSA Knee with OptimiZe later in the current year with commercial availability in the U.S. expected in the first quarter of 2026.
ROSA Knee with OptimiZe personalizes the surgeon's experience with customized intelligent surgical planning and new positioning, tracking and alignment features. The technology provides a simplified user interface that allows surgeons to choose the information they want to see, when they want to see it.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News