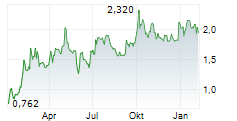
SynAct Pharma's Q325 results underscored the company's steady progress on its clinical strategy, led by the ongoing Phase IIb ADVANCE trial evaluating resomelagon as a first-line treatment for rheumatoid arthritis (RA). The study remains on track to complete enrolment by end-FY25, with top-line data anticipated in Q126. Operating performance reflected this acceleration, with operating expenses increasing 45.5% y-o-y (16.6% q-o-q) to SEK35.4m, reflecting higher R&D spend (+44.3% y-o-y; +18.6% q-o-q to SEK28.1m) as development activities ramp up. We anticipate a similar cost trajectory in Q425, followed by a moderation in 2026 after the Phase IIb readout. The SEK34.4m cash injection from warrant conversions in Q3 has strengthened the balance sheet with headroom to complete the ongoing Phase II studies, with further flexibility from the SEK30m credit facility to potentially initiate scoping exercises in other indications, such as RA-flares and virus-driven acute inflammatory conditions. Following our recent initiation, we reiterate our valuation of SEK1.97bn or SEK36.9/share.Den vollständigen Artikel lesen ...
© 2025 Edison Investment Research