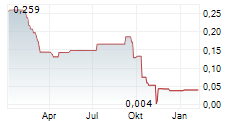
"With the planned study, we minimize the risks and increase the potential for fostrox to become the first approved treatment option in second-line liver cancer"
July - September
Financial summary for the quarter
- Net turnover amounted to SEK 0.9 (0.9) million.
- The loss before interest, tax, depreciation and amortization (EBITDA) amounted to SEK -12.9 (-35.1) million. Basic and diluted earnings per share amounted to SEK -0.13 (-0.30).
- Cash flow from operating activities amounted to SEK -14.1 (-33.4) million.
- Cash and cash equivalents at the end of the period amounted to SEK 23.5 (92.6) million.
Significant events during the quarter
- In July, Medivir received Notice of Allowance and subsequently a formal approval for fostrox plus lenvatinib combination patent by Japan Patent Office.
January - September
Financial summary for the period
- Net turnover amounted to SEK 3.0 (2.5) million.
- The loss before interest, tax, depreciation and amortization (EBITDA) amounted to SEK -48.0 (-98.4) million. Basic and diluted earnings per share amounted to SEK -0.45 (-0.85).
- Cash flow from operating activities amounted to SEK -67.0 (-94.8) million.
- Cash and cash equivalents at the end of the period amounted to SEK 23.5 (92.6) million.
Significant events after the period
- On October 8, it was announced that Medivir's board of directors has decided to carry out a fully guaranteed new share issue with preferential rights for existing shareholders of approximately SEK 151 million. The rights issue is conditional on approval at an extraordinary general meeting scheduled to be held on November 10, 2025.
- On October 23, an exclusive license agreement was signed with Canadian Biossil, Inc., giving Biossil global, exclusive development rights for remetinostat, a topical HDAC inhibitor in clinical phase that has shown positive phase 2 data in both basal cell carcinoma (BCC) and cutaneous T-cell lymphoma (CTCL).
Conference call for investors, analysts and the media
The Interim Report January - September 2025 will be presented by Medivir's CEO, Jens Lindberg.
Time: Thursday, November 6, 2025, at 15.00 (CET).
To call in to the conference - Please register here!
If you wish to participate via webcast - Please use this link!
The conference call will also be streamed via a link on the website: www.medivir.com/investors/calendar.
The presentation will be available on Medivir's website after completion of the conference.
CEO's message
With a focused study that enables faster result readout, we reduce risk and generate data that confirms the potential and value of fostrox.
Our belief in fostrox's potential to benefit patients with liver cancer is unwavering and was further strengthened by the final positive data we presented from the phase 1b/2a study with fostrox + Lenvima® in early 2025. The next step is a randomized study based on insights from in-depth discussions with investors and potential partners, to demonstrate the efficacy advantage of the combination compared to Lenvima alone. We have therefore chosen to first invest in a smaller, more focused, randomized study with the aim of demonstrating the difference between Lenvima and Lenvima plus fostrox. Confirmatory data that further strengthens the project allows us to better design a pivotal study in the next step, with reduced clinical risk.
The planned rights issue together with our excellent network within the HCC community provides us with the opportunity to collaborate with an experienced academic consortium to conduct a randomized, two-arm study with 30-50 patients in each arm. The goal is to demonstrate that fostrox in combination with Lenvima is superior to Lenvima alone in second-line advanced liver cancer.
To date, we have not seen any drug development projects in second-line liver cancer that have advanced to the point where they have stronger potential than fostrox + Lenvima. This was confirmed at the international ESMO congress in October where no progress was presented in second-line liver cancer. The fact that our study results are still better than what has been shown so far in second-line liver cancer makes it even more urgent to minimize the risks in the project and thereby increase the potential for fostrox to become the first approved treatment option.
Our already strong IP protection was further strengthened during the past quarter. In early July, we announced that the Japan Patent Office (JPO) had issued a Notice of Allowance and subsequently formally approved our patent application for patent protection for the combination of fostroxacitabine bralpamide (fostrox) and lenvatinib (Lenvima) for the treatment of hepatocellular carcinoma (HCC) and liver metastases from other types of cancer.
Business development continues to be a core component of Medivir's business model and after the end of the period we were able to present an exclusive license agreement with Biossil, Inc. The agreement gives Biossil global, exclusive development rights for remetinostat, a clinical-stage topical HDAC inhibitor that has shown positive Phase 2 data in both basal cell carcinoma (BCC) and cutaneous T-cell lymphoma (CTCL). Biossil is a Toronto-based, AI-native drug development company focused on developing novel therapies for heterogenous diseases with unmet medical needs. The terms of the agreement entitle Medivir to milestone payments of up to a total of approximately USD 60 million, assuming remetinostat is successfully developed and receives approval, in addition to mid-single digit royalties on future net sales.
I would like to emphasize again that our results to date show that there is a clear potential for fostrox + Lenvima to become the first approved drug treatment in second-line liver cancer - a market worth ~USD 2.5 billion annually. Through the study we are now planning, we reduce the risk and further increase the likelihood that fostrox will reach patients. We hope for continued support from existing shareholders in the planned rights issue that will enable this step.
I look forward to continue keeping you informed of Medivir's exciting developments.
Jens Lindberg
Chief Executive Officer
The Interim Report has been subject to auditors' review.
For additional information, please contact;
Magnus Christensen
Chief Financial Officer
M: +46 73 125 06 20
Email: Magnus.Christensen@medivir.com
About Medivir
Medivir develops innovative drugs with a focus on cancer where the unmet medical needs are high. The drug candidates are directed toward indication areas where available therapies are limited or missing and there are great opportunities to offer significant improvements to patients. Medivir is focusing on the development of fostroxacitabine bralpamide (fostrox), a drug candidate designed to selectively treat cancer cells in the liver and to minimize side effects. Collaborations and partnerships are important parts of Medivir's business model, and the drug development is conducted either by Medivir or in partnership. Medivir's share (ticker: MVIR) is listed on Nasdaq Stockholm's Small Cap list. www.medivir.com.