WASHINGTON (dpa-AFX) - Thermo Fisher Scientific Inc. (TMO) on Wednesday announced 510(k) clearance for its EXENT Analyzer and Immunoglobulin Isotypes (GAM) Assay, aimed at supporting faster diagnosis for patients with multiple myeloma and related disorders.
The EXENT System is an automated platform that detects and classifies M-proteins, abnormal antibodies produced by cancerous plasma cells, even at low concentrations, providing clinicians with reliable results.
With U.S. 510(k) clearance and clinical validation, the EXENT System is now available for clinical use in the U.S. and multiple international markets, including Australia, Belgium, Brazil, Canada, France, Germany, Italy, the Netherlands, New Zealand, Spain, Switzerland, and the U.K.
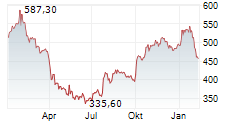
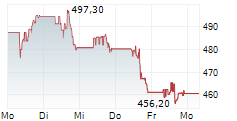
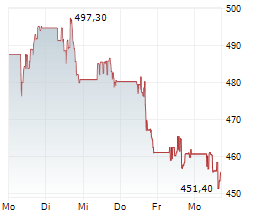
Thermo Fisher Scientific stock was at $586.81, up 0.06% in pre-market trading.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News