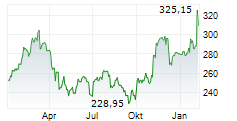
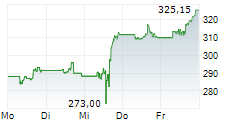
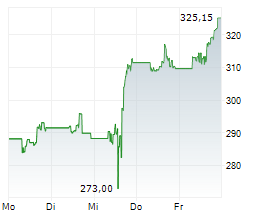
THOUSAND OAKS (dpa-AFX) - Amgen (AMGN) announced that the U.S. Food and Drug Administration has granted full approval to IMDELLTRA (tarlatamab-dlle) for the treatment of adult patients with extensive stage small cell lung cancer (ES-SCLC) with disease progression on or after platinum-based chemotherapy.
The decision to convert IMDELLTRA's prior accelerated approval to a full approval is based on data from the global Phase 3 DeLLphi-304 study.
Additionally, the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology (NCCN Guidelines) were recently updated to include tarlatamab as the only Category 1 preferred treatment option for adult patients with ES-SCLC with disease progression on or after platinum-based chemotherapy.
The global Phase 3 DeLLphi-304 study met its primary endpoint, demonstrating that IMDELLTRA reduced the risk of death by 40% and significantly extended median overall survival by more than five months compared to standard of care (SOC) chemotherapy as a treatment for patients with ES-SCLC who progressed on or after one line of platinum-based chemotherapy.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News