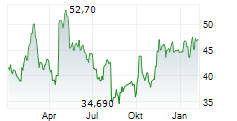
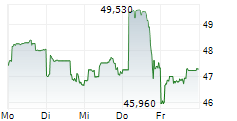
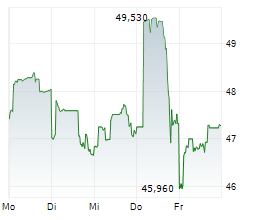
TOKYO (dpa-AFX) - Chugai Pharmaceutical Co., Ltd. (CHGCF.PK) on Tuesday reported positive results from its phase I/II TEIEN study evaluating the Port Delivery System with Ranibizumab (PDS) in patients with neovascular age-related macular degeneration (nAMD) and diabetic macular edema (DME). The PDS, an intraocular implant that provides long-term, sustained drug release, is designed to reduce the treatment burden associated with frequent eye injections.
According to Chugai, the PDS showed an efficacy trend consistent with outcomes seen in previous overseas clinical trials, with safety results also aligning with earlier data in both nAMD and DME patient populations.
'The current standard of care for nAMD and DME requires intraocular injections every 4 to 16 weeks, and a less burdensome treatment is desired. The PDS is expected to maintain long-term efficacy while reducing the treatment burden through an innovative intraocular implant that requires refills only once every 24 weeks,' said Dr. Osamu Okuda, Chugai's President and CEO.
Chugai said it will file a new drug application in Japan based on the TEIEN study findings and the results of Roche's overseas phase III studies in nAMD (Archway) and DME (Pagoda).
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News