WASHINGTON (dpa-AFX) - Denali Therapeutics Inc. (DNLI) and Royalty Pharma plc (RPRX) on Thursday announced a $275 million synthetic royalty funding agreement tied to future net sales of Tividenofusp alfa.
Tividenofusp alfa is Denali's lead investigational enzyme replacement therapy proposed for the treatment of mucopolysaccharidosis type II (MPS II, or Hunter syndrome). It is under FDA review, with a decision due on April 5, 2026.
The deal depends on various closing conditions, including Denali achieving U.S. FDA accelerated approval of Tividenofusp alfa. Royalty Pharma will make an initial payment of $200 million, with an additional payment of $75 million due if the European Medicines Agency approves Tividenofusp alfa by December 31, 2029.
In exchange, Royalty Pharma will earn a 9.25% royalty on worldwide net sales of tividenofusp alfa from Denali. The royalty payment is expected to terminate upon reaching a multiple of 3.0x, or 2.5x if achieved by the first quarter of 2039.
In January 2025, U.S. Food and Drug Administration has granted Fast Track Therapy designations to tividenofusp alfa for development in the treatment of MPS II. The European Medicines Agency has granted Priority Medicines designation to tividenofusp alfa in July 2023.
'We are delighted to partner with Denali and acquire a royalty on tividenofusp alfa, an innovative therapy that addresses a significant unmet need in the cognitive and physical manifestations of Hunter syndrome,' said Pablo Legorreta, Chief Executive Officer and Chairman of the Board of Royalty Pharma.
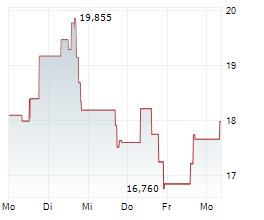
DNLI shares closed Wednesday's trade at $19.00 ,up 6.50%.
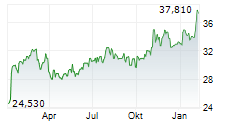
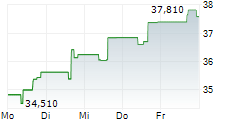
RPRX shares closed Wednesday's trade at $39.60,up 0.18%.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News