THOUSAND OAKS (dpa-AFX) - Amgen Inc. (AMGN), Friday, announced that the FDA has expanded UPLIZNA's label to include the treatment of generalized myasthenia gravis in adults who are positive for anti-acetylcholine receptor or anti-muscle-specific tyrosine kinase antibodies.
The approval was based on results from a phase 3 trial, dubbed MINT, in which UPLIZNA showed strong efficacy at 26 weeks in both anti-acetylcholine receptor (AChR+) and anti-muscle-specific tyrosine kinase (MuSK+) antibody positive, with AChR+ patients continuing to improve through 52 weeks in the study.
Myasthenia gravis is a rare autoimmune disorder that affects the neuromuscular system, leading to severe muscle weakness and extreme fatigue. There are two main types, namely ocular and generalized. About 85% of patients with myasthenia gravis have the generalized form, or gMG.
Antibodies against AChR (Acetylcholine receptor) and MuSK (muscle-specific tyrosine kinase) are detected in patients with myasthenia gravis. Approximately 85% of patients with myasthenia gravis have detectable antibodies against AChR, and approximately 7% have detectable antibodies against MuSK.
This marks the third approved indication for UPLIZNA and makes it the first and only CD19-targeted B-cell therapy approved for adults with anti-AChR and anti-MuSK antibody-positive generalised myasthenia gravis.
UPLIZNA is already approved in the U.S. for the treatment of adult patients with anti-aquaporin-4 (AQP4) antibody-positive neuromyelitis optica spectrum disorder (NMOSD) and for the treatment of adult patients with Immunoglobulin G4-related disease (IgG4-RD).
Sales of UPLIZNA were $379 million for the full year 2024, and $422?million in the first nine months of 2025.
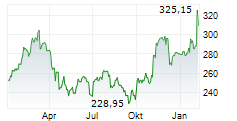
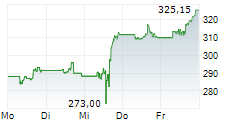
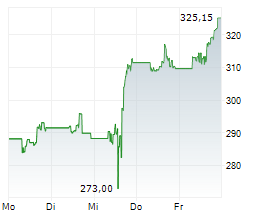
On Thursday, AMGN closed trading at $317.38, up 0.63%.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News