WASHINGTON (dpa-AFX) - Incyte Corp. (INCY) announced on Thursday that the European Commission has approved Minjuvi in combination with lenalidomide and rituximab for the treatment of adult patients with relapsed or refractory follicular lymphoma (FL) (Grade 1-3a) after at least one line of systemic therapy.
The regulatory agency's decision was based on positive data from a Phase 3 inMIND trial that showed Minjuvi, combined with rituximab and lenalidomide, met its primary endpoint of progression-free survival.
Last month, the European Medicines Agency's Committee for Medicinal Products for Human Use (CHMP) issued a positive opinion recommending the approval of Minjuvi in combination with lenalidomide and rituximab for the treatment of adult patients with relapsed or refractory follicular lymphoma (FL) (Grade 1-3a) after at least one line of systemic therapy.
Follicular lymphoma (FL) is the most common type of slow-growing blood cancer that starts in B cells, a kind of white blood cell and represents about 30% of non-Hodgkin lymphoma cases globally.
Minjuvi, chemically known as tafasitamab-cxix, is a humanised Fc-modified cytolytic CD19-targeting monoclonal antibody. Incyte licensed the exclusive worldwide rights to develop and commercialise tafasitamab from Xencor, Inc.
Notably, earlier in 2021, the EC approved Minjuvi in combination with lenalidomide for the indication of relapsed or refractory diffuse large B-cell lymphoma.
In the U.S, the drug is approved under the brand name Monjuvi. It is approved for use with lenalidomide to treat adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL), and in combination with rituximab and lenalidomide for adults with relapsed or refractory follicular lymphoma.
In the third quarter of 2025, Minjuvi/Monjuvi sales improved 34% to $41.99 million from $31.44 million in the prior year.
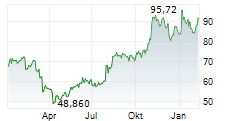
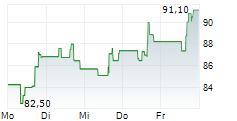
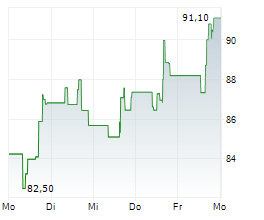
INCY closed Wednesday's trade at $97.63, up 0.62%.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News