MADRID (dpa-AFX) - Biotest AG, Friday announced that its parent company Grifols, S.A. (GRFS) has received approval from the U.S. Food and Drug Administration for Fesilty, a human fibrinogen product developed for the treatment of acute bleeding episodes in pediatric and adult patients with congenital fibrinogen deficiency.
With this approval, Grifols will be able to launch this product in the first half of 2026 in the U.S.
Dr. Jörg Schüttrumpf, Chief Executive Officer of Biotest AG, said, 'We are proud that our plasma expertise contributes directly to expanding patient access to life-saving fibrinogen therapies in the treatment of critical conditions worldwide.'
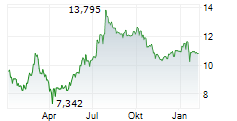
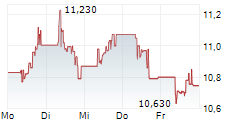
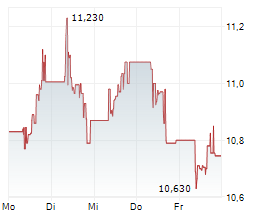
Currently, GRFS is trading at $8.99, up 0.84 percent on the Nasdaq.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News