WESTON (dpa-AFX) - Biogen Inc. (BIIB) announced that the European Commission has granted marketing authorization for a high dose regimen of SPINRAZA (nusinersen) which is comprised of 50 mg/5 mL and 28 mg/5 mL doses for the treatment of 5q spinal muscular atrophy (SMA).
5q SMA is the most common form of the disease and represents approximately 95% of all SMA cases. The SPINRAZA European Union marketing authorization has been updated to include the high dose regimen. The new high dose regimen comprises a more rapid loading phase, two 50 mg loading doses administered 14 days apart and 28 mg maintenance dose injections every four months thereafter. Individuals transitioning from the 12 mg dose will receive one 50 mg dose in place of their next 12 mg dose, followed by 28 mg maintenance doses every four months thereafter. SPINRAZA is for intrathecal use by lumbar puncture by health care professionals experienced in performing lumbar punctures.
The high dose regimen of SPINRAZA is also approved in Japan and is under review with the U.S. Food and Drug Administration (FDA) with a decision expected by April 3, 2026. Biogen is working with regulatory authorities around the world to progress this additional dosing option for people living with SMA.
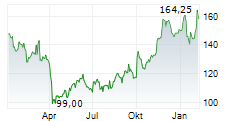
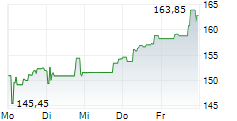
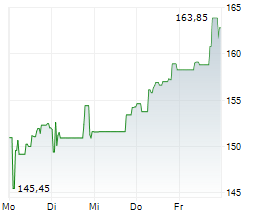
BIIB closed trading at $185.63, down $1.99 or 1.06% as of 4:00 PM EST. In overnight trading at 9:30 PM EST, the stock slipped further to $185.20, representing a decline of $0.43 or 0.23%.
For More Such Health News, visit rttnews.com.
Copyright(c) 2026 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2026 AFX News