PARIS (dpa-AFX) - Ipsen (IPSEY, IPN.PA) announced that the U.S. Food and Drug Administration has granted Breakthrough Therapy Designation for IPN60340 in combination with venetoclax and azacitidine (Ven-Aza) in first line unfit acute myeloid leukemia, an aggressive blood cancer affecting older adults.
IPN60340 is an investigational first-in-class monoclonal antibody targeting BTN3A, a key immune-regulatory molecule broadly expressed across cancer. Breakthrough Therapy Designation is intended to expedite the development and review of medicines for serious or life-threatening conditions with evidence of a substantial clinical improvement. IPN60340 previously received Orphan Drug Designations from the U.S. Food and Drug Administration and European Medicines Agency in July 2025.
The company said it looks forward to discussing the design of the Phase II/III development plans with the FDA for IPN60340 in the first-half of 2026.
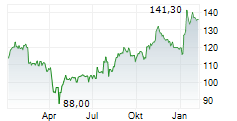
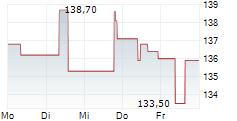
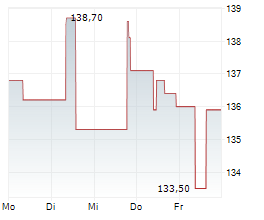
Ipsen closed Tuesday's regular trading in Paris at 127.50 euros, representing a decline of 1.80 euros or 1.39%.
For More Such Health News, visit rttnews.com.
Copyright(c) 2026 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2026 AFX News