BASEL (dpa-AFX) - Novartis announced that the U.S. Food and Drug Administration has granted Breakthrough Therapy designation to ianalumab for Sjögren's disease, the second most prevalent rheumatic autoimmune disease.
Ianalumab is a fully human monoclonal antibody with a novel dual mechanism of action that depletes B-cells and inhibits their activation and survival via BAFF-R blockade.
The company noted that it plans to submit ianalumab for regulatory approval globally starting in early 2026. If approved, ianalumab would become the first targeted treatment for patients with Sjögren's disease.
Sjögren's disease is a serious, progressive, autoimmune condition that affects multiple organs causing a wide spectrum of symptoms such as dryness, fatigue, pain, and an increased risk of lymphoma, which can carry a significant burden and impact on quality of life.
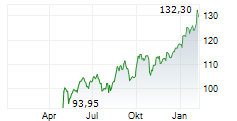
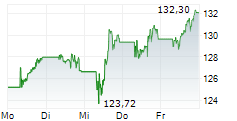
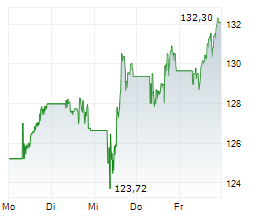
NVS closed Friday's regular trading session at $144.34, rising $1.19 or 0.83% as of 4:00 PM EST. In after-hours trading, the stock inched up to $144.35, a modest gain of $0.01 or 0.01%.
For More Such Health News, visit rttnews.com.
Copyright(c) 2026 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2026 AFX News