WASHINGTON (dpa-AFX) - Viatris Inc. (VTRS), a healthcare company, Tuesday announced the launch of Inpefa or sotagliflozin in the United Arab Emirates or UAE, which is the first country within the Viatris territories to commercialize this treatment.
The company said future launches are expected in multiple countries over the next several years, supporting Viatris' strategy to expand access to the treatment.
Inpefa is the first and only dual SGLT1/2 inhibitor approved for the treatment of heart failure. This drug is already approved in the US to reduce the risk of cardiovascular death, hospitalization for heart failure, and urgent heart failure visits in adults with heart failure or type 2 diabetes mellitus, chronic kidney disease, and other cardiovascular risk factors.
The approval of Inpefa is based on two pivotal Phase 3 trials, SOLOIST-WHF and SCORED which enrolled more than 11,800 patients globally.
As per the company, in SOLOIST-WHF, Inpefa reduced the composite risk of heart failure hospitalization, urgent visits, and cardiovascular death by 33 percent versus placebo in patients recently hospitalized for worsening heart failure, with benefits increasing up to 51 percent when initiated prior to discharge and evident within 30 days post-discharge.
In SCORED, among patients with type 2 diabetes and chronic kidney disease with additional cardiovascular risk factors both with and without heart failure, Inpefa achieved a 25 percent reduction in the same composite endpoint.
Further, the drug was consistent with its dual SGLT1 and SGLT2 inhibition, MACE defined as CV death, non-fatal MI, and non-fatal stroke was reduced by 23 percent, with benefits observed as early as 94 days. It is the first SGLT inhibitor to show a significant reduction in both MI by 32 percent and stroke by 34 percent.
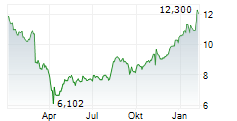
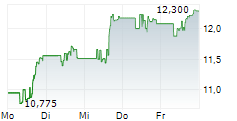
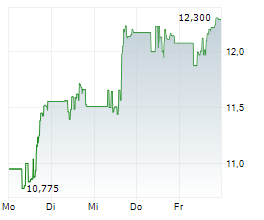
In pre-market activity, VTRS shares were trading at $12.73, down 1.01% on the Nasdaq.
Copyright(c) 2026 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2026 AFX News