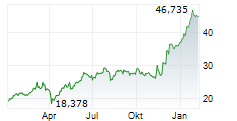
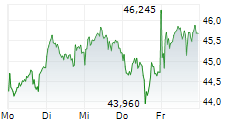
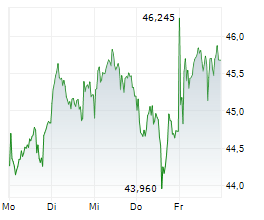
LEVERKUSEN (dpa-AFX) - Bayer AG (BYR.L, BAY.MI, BAYN.DE) and its independently operated subsidiary BlueRock Therapeutics LP announced that its investigational cell therapy, OpCT-001, has received Orphan Drug Designation from the FDA for treating retinitis pigmentosa. OpCT-001 is an investigational induced pluripotent stem cell-derived cell therapy being tested in a Phase 1/2a clinical study for the treatment of primary photoreceptor diseaseses.
Amit Rakhit, BlueRock's Chief Medical Officer, said: 'We believe that OpCT-001 has great promise as a potential therapeutic option for restoring vision in people living with retinitis pigmentosa and other primary photoreceptor diseases and look forward to continuing to work with the FDA on its clinical development.'
For More Such Health News, visit rttnews.com.
Copyright(c) 2026 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2026 AFX News