WESTON (dpa-AFX) - Biogen, Inc. (BIIB) announced Wednesday that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Therapy Designation for litifilimab (BIIB059) for the treatment of cutaneous lupus erythematosus (CLE).
Litifilimab is a first in-class, humanized IgG1 monoclonal antibody (mAb) targeting blood dendritic cell antigen 2 (BDCA2). CLE is a chronic autoimmune disease affecting the skin that currently has no targeted treatments.
The designation is intended to expedite the development and review of drugs for serious conditions, and is based on the totality of litifilimab data, including the results from the Phase 2 LILAC study.
Biogen is continuing to evaluate the efficacy and safety of litifilimab in the AMETHYST Phase 3 study, with a data readout expected in 2027.
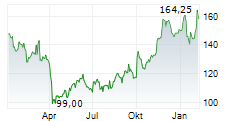
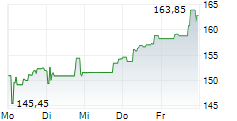
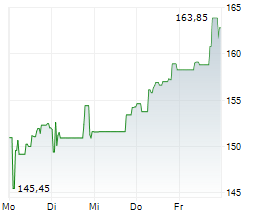
In Wednesday's pre-market trading, BIIB is trading on the Nasdaq at $175.24, up $1.12 or 0.64 percent.
Copyright(c) 2026 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2026 AFX News