WESTON (dpa-AFX) - Biogen (BIIB) announced that Nature Medicine published results from the Phase 2/3 DEVOTE study evaluating the high-dose regimen of nusinersen, comprised of 50 mg/5 mL loading and 28 mg/5 mL maintenance doses, in spinal muscular atrophy. The company said the results showed the safety and effectiveness of the high-dose regimen of nusinersen across a broad range of people living with SMA, irrespective of age, prior treatment experience, and baseline functional status.
The high-dose regimen of SPINRAZA or nusinersen is approved in the European Union and Japan. The high-dose regimen is under review with the FDA and has a PDUFA action date of April 3.
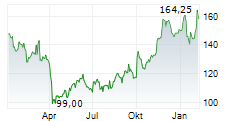
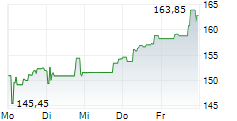
In pre-market trading on NasdaqGS, Biogen shares are up 0.53 percent to $177.69.
For More Such Health News, visit rttnews.com.
Copyright(c) 2026 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2026 AFX News