| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
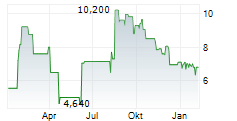
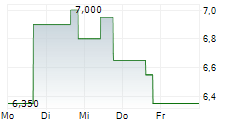
| 11,600 | 11,900 | 14:23 | |
| 11,600 | 11,800 | 12:58 |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 18.02. | Elicio Therapeutics Inc.: Elicio Therapeutics Reports Inducement Grants | 8 | GlobeNewswire (USA) | ||
| 16.01. | Elicio Therapeutics Inc.: Elicio Therapeutics Reports Inducement Grants | 10 | GlobeNewswire (USA) | ||
| 19.11.25 | Elicio Therapeutics appoints Marc Wolfgang as chief technology officer | 1 | Investing.com | ||
| 19.11.25 | Elicio Therapeutics Inc.: Elicio Therapeutics Appoints Veteran CMC and Technical Operations Executive Marc J. Wolfgang as Chief Technology Officer | 415 | GlobeNewswire (Europe) | BOSTON, Nov. 19, 2025 (GLOBE NEWSWIRE) -- Elicio Therapeutics, Inc. (Nasdaq: ELTX, "Elicio" or the "Company"), a clinical-stage biotechnology company developing a pipeline of novel immunotherapies... ► Artikel lesen | |
| 13.11.25 | Elicio Therapeutics GAAP EPS of -$0.60 misses by $0.12 | 2 | Seeking Alpha | ||
| 13.11.25 | Elicio Therapeutics Inc.: Elicio Therapeutics Reports Third Quarter 2025 Financial Results and Provides Corporate Updates | 195 | GlobeNewswire (Europe) | In the ongoing Phase 2 AMPLIFY-7P study evaluating ELI-002 7P in patients with pancreatic ductal adenocarcinoma ("PDAC"), fewer disease progressions and deaths than projected have been observed as... ► Artikel lesen | |
| 07.11.25 | Elicio meldet starke Immunantworten in Phase-2-Studie zu KRAS-Krebsimpfstoff | 1 | Investing.com Deutsch | ||
| ELICIO THERAPEUTICS Aktie jetzt für 0€ handeln | |||||
| 07.11.25 | Elicio Therapeutics Inc.: Elicio Therapeutics Reports Robust, Cytolytic mKRAS-Specific T Cell Responses Across Diverse Patient HLA in Ongoing Phase 2 AMPLIFY-7P Trial of ELI-002 7P and New ELI-004 Preclinical Data at SITC | 235 | GlobeNewswire (Europe) | SITC abstracts highlight updated immunogenicity results from the Phase 2 AMPLIFY-7P trial of ELI-002 7P and preclinical data on the use of intratumoral ELI-004 (AMP-CpG) for solid tumor immunotherapyELI-002... ► Artikel lesen | |
| 27.10.25 | Krebstherapie von Elicio zeigt breite Immunantwort bei diversen HLA-Typen | 1 | Investing.com Deutsch | ||
| 27.10.25 | Elicio's cancer therapy shows immune response across diverse HLA types | 2 | Investing.com | ||
| 18.09.25 | Elicio Therapeutics Inc.: Elicio Therapeutics Reports Inducement Grants | 228 | GlobeNewswire (Europe) | BOSTON, Sept. 18, 2025 (GLOBE NEWSWIRE) -- Elicio Therapeutics, Inc. (Nasdaq: ELTX, "Elicio" or the "Company"), a clinical-stage biotechnology company developing a pipeline of novel immunotherapies... ► Artikel lesen | |
| 17.09.25 | Elicio Reports Strong T Cell Responses In Phase 2 Trial Of Cancer Vaccine | 1 | RTTNews | ||
| 17.09.25 | Elicio: Krebsimpfstoff ELI-002 löst bei 99 % der Patienten starke Immunantwort aus | 2 | Investing.com Deutsch | ||
| 17.09.25 | Elicio's cancer vaccine ELI-002 shows strong immune response in 99% of patients | 2 | Investing.com | ||
| 17.09.25 | Elicio Therapeutics, Inc. - 8-K, Current Report | 1 | SEC Filings | ||
| 17.09.25 | Elicio Therapeutics Inc.: Elicio Therapeutics Reports ELI-002 7P Achieved Robust mKRAS-Specific T Cell Responses in 99% of Evaluable Patients in Ongoing Phase 2 AMPLIFY-7P Trial | 228 | GlobeNewswire (Europe) | ELI-002 induced mKRAS-specific T cell responses in 99% of evaluable patients (89 of 90) who were treated with the investigational vaccine immunotherapyRobust mKRAS-specific T cell responses were observed... ► Artikel lesen | |
| 12.08.25 | Elicio Therapeutics Inc.: Elicio Therapeutics Announces Publication of ELI-002 Updated AMPLIFY-201 Phase 1 Follow-up Data in Nature Medicine for Minimal Residual Disease ("MRD") Positive, Adjuvant-Stage Patients | 237 | GlobeNewswire (Europe) | At extended median follow-up of 19.7 months, median overall survival ("OS") increased from 16.33 to 28.94 months Clinical efficacy correlated with the magnitude of T cell responses specific to mutant-KRAS... ► Artikel lesen | |
| 07.08.25 | Elicio Therapeutics Inc.: Elicio Therapeutics Reports Second Quarter 2025 Financial Results and Provides Corporate Updates | 355 | GlobeNewswire (Europe) | Recent positive recommendation by the Independent Data Monitoring Committee ("IDMC") to continue ELI-002 7P randomized Phase 2 study in pancreatic cancer without modifications to final analysis Event-driven... ► Artikel lesen | |
| 16.04.25 | Elicio Therapeutics Inc.: Elicio Therapeutics Reports Inducement Grant to New Chief Strategy and Financial Officer and Other New Hires | 332 | GlobeNewswire (Europe) | BOSTON, April 16, 2025 (GLOBE NEWSWIRE) -- Elicio Therapeutics, Inc. (Nasdaq: ELTX, "Elicio Therapeutics" or "Elicio"), a clinical-stage biotechnology company developing a pipeline of novel immunotherapies... ► Artikel lesen | |
| 31.03.25 | Elicio Therapeutics Inc.: Elicio Therapeutics Reports 2024 Financial Results and Provides Corporate Updates | 457 | GlobeNewswire (Europe) | Completed enrollment of Phase 2 AMPLIFY-7P randomized study; disease-free survival ("DFS") event-driven interim analysis expected in Q3 2025 Aligned with U.S. Food and Drug Administration ("FDA")... ► Artikel lesen |
20 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| NANOREPRO | 1,575 | +2,61 % | NanoRepro plant Kapitalerhöhung - Kaltplasmagerät startet am Markt | Die NanoRepro AG erhöht ihr Grundkapital unter teilweiser Ausnutzung des Genehmigten Kapitals 2024 um bis zu 1,29 Millionen Aktien auf bis zu 14,19 Millionen Aktien. Vorstand und Aufsichtsrat des Unternehmens... ► Artikel lesen | |
| OCUGEN | 1,625 | -1,87 % | Ocugen Announces Phase 3 liMeliGhT Enrollment Completion for OCU400, a Novel Modifier Gene Therapy for Broad Retinitis Pigmentosa | Enrollment for liMeliGhT, the first and largest gene therapy registrational trial for broad retinitis pigmentosa (RP) patients, was completed, reflecting strong interest from investigators and patientsTopline... ► Artikel lesen | |
| VIKING THERAPEUTICS | 27,810 | -2,47 % | Viking Therapeutics: The High-Stakes Weight Loss Contender | ||
| COSCIENS BIOPHARMA | 1,480 | 0,00 % | COSCIENS Biopharma Inc. Announces Leadership Change | TORONTO, ONTARIO, Nov. 14, 2025 (GLOBE NEWSWIRE) -- COSCIENS Biopharma Inc. (TSX: CSCI) (FINRA: CSCIF) ("COSCIENS" or the "Company"), a life science company focused on natural ingredients and pharmaceutical... ► Artikel lesen | |
| SCORPIUS | 0,070 | 0,00 % | Scorpius Holdings, Inc. - 8-K, Current Report | ||
| BIOCRYST PHARMACEUTICALS | 7,244 | -1,71 % | BioCryst Pharmaceuticals, Inc.: BioCryst Reports Full Year 2025 Financial Results and Provides Business Update | -Full year 2025 ORLADEYO® net revenue of $601.8 million (+38% y-o-y; +43% y-o-y excluding European ORLADEYO revenue following the sale of the European ORLADEYO business to Neopharmed Gentili S.p.A.... ► Artikel lesen | |
| IBIO | 2,440 | +5,17 % | Jones Trading initiates iBio stock with buy rating on obesity platform | ||
| VAXART | 0,560 | +9,80 % | Vaxart, Inc.: Vaxart Provides Business Update and Reports Third Quarter 2025 Financial Results | Entered into an exclusive license agreement with Dynavax for the Company's COVID-19 oral pill vaccine candidate for potential cumulative proceeds of up to $700 million plus royalties Completed enrollment... ► Artikel lesen | |
| ARBUTUS BIOPHARMA | 3,920 | -2,20 % | Arbutus Biopharma Corporation: Arbutus Reports Third Quarter 2025 Financial Results and Provides Corporate Update | Strong financial position with cash, cash equivalents and marketable securities of $93.7M Moderna litigation U.S. trial scheduled for March 2026;Favorable claim construction ruling in Pfizer-BioNTech... ► Artikel lesen | |
| REDHILL BIOPHARMA | 0,912 | -5,98 % | RedHill Biopharma Ltd. - 6-K, Report of foreign issuer | ||
| CARDIFF ONCOLOGY | 1,592 | -4,33 % | Cardiff Oncology, Inc.: Cardiff Oncology Reports Full Year 2025 Results and Provides Business Update | Reported positive update from Phase 2 CRDF-004 trial in first-line RAS-mutated mCRC, with the 30 mg onvansertib + FOLFIRI/bev arm demonstrating:• Robust ORR of 72.2% (vs 43.2% with combined SoC of... ► Artikel lesen | |
| MANNKIND | 2,601 | +0,19 % | H.C. Wainwright cuts MannKind stock price target on Tyvaso outlook | ||
| ALDEYRA | 4,834 | +0,65 % | Aldeyra Therapeutics, Inc. - S-8, Securities to be offered to employees in employee benefit plans | ||
| TRAWS PHARMA | 1,650 | 0,00 % | Traws Pharma, Inc.: Traws Pharma Completes Analysis of Ratutrelvir Clinical Study in PAXLOVID-Eligible and Ineligible COVID-19 Patients and Provides Updates for Additional Indication for Tivoxavir Marboxil as a Prophylactic Treatment for Seasonal ... | Completed clinical results with ratutrelvir confirm a differentiated profile versus PAXLOVID- with fewer adverse events and no viral rebounds with equivalent time to sustained symptom resolution... ► Artikel lesen | |
| COHERUS ONCOLOGY | 1,358 | -4,10 % | Coherus Oncology, Inc.: Coherus Oncology to Report Full Year and Fourth Quarter 2025 Financial Results on March 9, 2026 |
ELICIO THERAPEUTICS INC gehört der Branche Biotechnologie an. Der aktuelle Kurs liegt bei 11,800 Euro und damit -0,84 % im Minus.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu ELICIO THERAPEUTICS INC finden Sie auf Wallstreet Online.