- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| INMOLECULE NANOTECH Aktie jetzt für 0€ handeln | |||||
| 26.09.25 | XFRA NEW INSTRUMENTS AVAILABLE ON 26.09.2025 | 580 | Xetra Newsboard | The following instruments on XETRA do have their first trading 26.09.2025 Die folgenden Instrumente in XETRA haben ihren ersten Handelstag 26.09.2025
Aktien
1 SE0023135298 NOBA Bank Group AB
2... ► Artikel lesen |
1 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| QIAGEN | 40,700 | -1,44 % | Shortseller-Positionen aktuell: freenet, GFT, HelloFresh, Hypoport, Puma, Qiagen, Redcare Pharmacy | Wer Aktien leerverkauft, also auf fallende Kurse spekuliert (Shortselling), unterliegt bestimmten Transparenzpflichten. Aktuelle Meldungen über Leerverkäufe geben Aufschluss darüber. Diese Vorgaben... ► Artikel lesen | |
| KYMERA THERAPEUTICS | 90,10 | 0,00 % | Kymera auf TD Cowen Konferenz: Strategische Einblicke in Medikamenten-Pipeline und Partnerschaften | ||
| VERA THERAPEUTICS | 40,420 | 0,00 % | Vera Therapeutics, Inc. - 10-K, Annual Report | ||
| ADMA BIOLOGICS | 16,580 | 0,00 % | ADMA Biologics Announces $200 Mln Capital Return Plan | ||
| BIONTECH | 87,50 | -4,94 % | BioNTech SE: BioNTech veröffentlicht am 10. März 2026 Ergebnisse für das vierte Quartal und das Geschäftsjahr 2025 und informiert über operativen Fortschritt | MAINZ, Deutschland, 24. Februar 2026 (GLOBE NEWSWIRE) -- BioNTech SE (Nasdaq: BNTX, "BioNTech" oder "das Unternehmen") wird am Dienstag, den 10. März 2026, die Ergebnisse für das vierte Quartal und... ► Artikel lesen | |
| EVOTEC | 5,304 | -6,72 % | Evotec Aktie vor dem Durchbruch? Diesmal ist etwas anders... | Die Evotec Aktie hat zuletzt ein wichtiges Muster bestätigt: Wiederholt scheiterte die Biotech-Aktie aus Hamburg mit Breakversuchen an der 200-Tage-Linie. Nach mehr oder weniger starken Ausbrüchen hierüber... ► Artikel lesen | |
| TREVI THERAPEUTICS | 12,570 | 0,00 % | Trevi Therapeutics, Inc.: Trevi Therapeutics Announces Appointment of David Hastings as Chief Financial Officer | Experienced biotech CFO to lead financial strategy and contribute to the Company's next stage of growth
NEW HAVEN, Conn., Dec. 4, 2025 /PRNewswire/ -- Trevi Therapeutics... ► Artikel lesen | |
| APOGEE THERAPEUTICS | 70,92 | 0,00 % | RBC Capital lowers Apogee Therapeutics stock price target on dosing outlook | ||
| PRAXIS PRECISION MEDICINES | 330,19 | 0,00 % | Praxis Precision Medicines, Inc.: Praxis Precision Medicines Provides Corporate Update and Reports Fourth Quarter and Full-Year 2025 Financial Results | Two new drug applications (NDA) for ulixacaltamide in essential tremor (ET) and for relutrigine in SCN2A and SCN8A developmental and epileptic encephalopathies (DEEs) have been submitted to the U.S.... ► Artikel lesen | |
| TYRA BIOSCIENCES | 32,520 | 0,00 % | Tyra Biosciences Reports Fourth Quarter and Full-Year 2025 Financial Results and Recent Highlights | - Launched "dabogratinib 3x3" strategy to pursue 3 late-stage clinical studies in LG-UTUC, IR NMIBC and ACH, 3 potential blockbuster indications -
- Interim Ph2... ► Artikel lesen | |
| MOONLAKE IMMUNOTHERAPEUTICS | 17,680 | 0,00 % | Cantor Fitzgerald reiterates Moonlake stock rating on axSpA potential | ||
| KINIKSA PHARMACEUTICALS | 46,040 | 0,00 % | Kiniksa Pharmaceuticals International, Plc: Kiniksa Pharmaceuticals Reports Fourth Quarter and Full Year 2025 Financial Results and Recent Portfolio Execution | - ARCALYST- (rilonacept) Q4 2025 and full year 2025 net product revenue of $202.1 million and $677.6 million, respectively -- ARCALYST 2026 net product revenue expected to be $900 - $920 million... ► Artikel lesen | |
| COGENT BIOSCIENCES | 38,890 | 0,00 % | Cogent Biosciences, Inc.: Cogent Biosciences Highlights Additional Data with Six Bezuclastinib Posters from SUMMIT Trial at 2026 AAAAI Annual Meeting | Bezuclastinib mean TSS reduction deepens to -32.0 points at 48 weeks of treatment with further improvement shown across all measured symptoms99% of patients achieve >50% reduction in serum tryptase... ► Artikel lesen | |
| DYNE THERAPEUTICS | 16,145 | +3,36 % | Dyne Therapeutics, Inc.: Dyne Therapeutics Reports Fourth Quarter and Full Year 2025 Financial Results and Recent Business Highlights | - Planned submission for U.S. Accelerated Approval of z-rostudirsen on track for Q2 2026; potential launch in Q1 2027 - - Positive topline results reported from Phase 1/2 DELIVER trial of z-rostudirsen... ► Artikel lesen | |
| SUMMIT THERAPEUTICS | 16,190 | 0,00 % | H.C. Wainwright cuts Summit Therapeutics stock price target on China trial timing uncertainty |
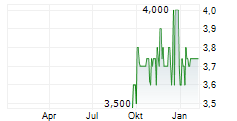
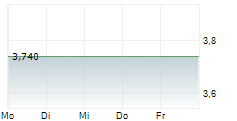
INMOLECULE NANOTECH SA gehört der Branche Biotechnologie an. Die relative Kursveränderung beträgt aktuell 0,00 % bei einem Aktienkurs von 3,740 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu INMOLECULE NANOTECH SA finden Sie auf Wallstreet Online.