- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Mi | Tyra Biosciences platziert Aktienpaket im Wert von 126 Mio. US-Dollar in Block-Trade | 2 | Investing.com Deutsch | ||
| Mi | Tyra Biosciences: Aktie auf Rekordhoch nach Kursrally von über 200 % | 4 | Investing.com Deutsch | ||
| Di | Tyra Biosciences: H.C. Wainwright bekräftigt "Buy"-Rating und Kursziel von 45 Dollar | 1 | Investing.com Deutsch | ||
| Mo | Tyra Biosciences GAAP EPS of -$0.57 misses by $0.04 | 1 | Seeking Alpha | ||
| Mo | Tyra Biosciences, Inc. - 10-K, Annual Report | 1 | SEC Filings | ||
| Mo | Tyra Biosciences, Inc. - 8-K, Current Report | 1 | SEC Filings | ||
| Mo | Tyra Biosciences Reports Fourth Quarter and Full-Year 2025 Financial Results and Recent Highlights | 92 | PR Newswire | - Launched "dabogratinib 3x3" strategy to pursue 3 late-stage clinical studies in LG-UTUC, IR NMIBC and ACH, 3 potential blockbuster indications -
- Interim Ph2... ► Artikel lesen | |
| TYRA BIOSCIENCES Aktie jetzt für 0€ handeln | |||||
| 10.02. | William Blair startet Coverage für Tyra Biosciences mit "Outperform"-Rating | 2 | Investing.com Deutsch | ||
| 10.02. | William Blair initiates coverage on Tyra Biosciences stock with Outperform rating | 1 | Investing.com | ||
| 28.01. | Tyra Biosciences stock price target raised to $55 from $35 at Raymond James | 4 | Investing.com | ||
| 23.01. | Tyra Biosciences: Piper Sandler erhöht Kursziel wegen Wachstumsaussichten auf 42 US-Dollar | 1 | Investing.com Deutsch | ||
| 23.01. | Piper Sandler raises Tyra Biosciences stock price target to $42 on growth outlook | 2 | Investing.com | ||
| 16.12.25 | Wedbush raises Tyra Biosciences stock price target to $37 on pipeline potential | 1 | Investing.com | ||
| 04.12.25 | Goldman Sachs initiates coverage of Tyra Biosciences stock with Early-Stage Biotech rating | 1 | Investing.com | ||
| 01.12.25 | Tyra Biosciences appoints two key executives to leadership team | 1 | Investing.com | ||
| 01.12.25 | Tyra Biosciences Strengthens Leadership Team with Appointments of Bhavesh Ashar as Chief Operating Officer and Heather Faulds as Chief Regulatory Officer | 194 | PR Newswire | CARLSBAD, Calif., Dec. 1, 2025 /PRNewswire/ -- Tyra Biosciences, Inc. (Nasdaq: TYRA), a clinical-stage biotechnology company focused on developing next-generation... ► Artikel lesen | |
| 01.12.25 | Tyra Biosciences, Inc. - 8-K, Current Report | - | SEC Filings | ||
| 06.11.25 | Tyra Biosciences GAAP EPS of -$0.50 beats by $0.05 | 1 | Seeking Alpha | ||
| 05.11.25 | Tyra Biosciences Reports Third Quarter 2025 Financial Results and Highlights | 157 | PR Newswire | - Dosed first patients with dabogratinib in BEACH301 and SURF302; interim results from both studies expected in 2026 -
- Expanded development of dabogratinib... ► Artikel lesen | |
| 10.09.25 | Oppenheimer raises Tyra Biosciences stock price target to $36 on market potential | 3 | Investing.com |
1 2 Weiter >>
25 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| DAY ONE BIOPHARMACEUTICALS | 21,200 | +65,75 % | Servier; Day One Biopharmaceuticals: Servier and Day One Biopharmaceuticals announce acquisition to expand Servier's rare oncology portfolio | Acquisition positions Servier as a leader in pediatric low-grade glioma and expands its pipeline with programs targeting adult and pediatric cancers with high unmet needs. Transaction represents... ► Artikel lesen | |
| QIAGEN | 39,095 | -3,59 % | Shortseller-Positionen aktuell: Auto1, Energiekontor, freenet, Gerresheimer, Puma, Qiagen, TeamViewer | Wer Aktien leerverkauft, also auf fallende Kurse spekuliert (Shortselling), unterliegt bestimmten Transparenzpflichten. Aktuelle Meldungen über Leerverkäufe geben Aufschluss darüber. Diese Vorgaben... ► Artikel lesen | |
| TANGO THERAPEUTICS | 16,950 | +0,77 % | Erasca, Inc.: Erasca and Tango Therapeutics Enter into Clinical Collaboration to Evaluate Combination of ERAS-0015 and Vopimetostat | ERAS-0015, a pan-RAS molecular glue, will be evaluated in combination with PRMT5 inhibitor vopimetostat Tango will sponsor the clinical trial and Erasca will supply ERAS-0015 at no cost SAN DIEGO... ► Artikel lesen | |
| IMMUNOME | 21,040 | +0,43 % | Immunome: Q4 Earnings Insights | ||
| KINIKSA PHARMACEUTICALS | 46,160 | +0,26 % | Kiniksa Pharmaceuticals International, Plc: Kiniksa Pharmaceuticals Reports Fourth Quarter and Full Year 2025 Financial Results and Recent Portfolio Execution | - ARCALYST- (rilonacept) Q4 2025 and full year 2025 net product revenue of $202.1 million and $677.6 million, respectively -- ARCALYST 2026 net product revenue expected to be $900 - $920 million... ► Artikel lesen | |
| ARCELLX | 114,33 | -0,03 % | Arcellx stock rating cut to Hold by TD Cowen on Gilead deal | ||
| ARCUTIS BIOTHERAPEUTICS | 24,000 | +0,17 % | Arcutis Biotherapeutics, Inc.: Arcutis Announces Promotion of Mas Matsuda to Executive Vice President and Chief Legal Officer to Support Next Phase of Growth | WESTLAKE VILLAGE, Calif., March 05, 2026 (GLOBE NEWSWIRE) -- Arcutis Biotherapeutics, Inc. (Nasdaq: ARQT), a commercial-stage biopharmaceutical company focused on developing meaningful innovations... ► Artikel lesen | |
| BIONTECH | 87,05 | -0,51 % | BioNTech-Konkurrent Moderna: Satter Kurssprung - jetzt noch einsteigen? | Die Aktie von Moderna hat zuletzt erneut kräftigen Rückenwind erhalten. Die Korrektur seit Ende Januar wurde damit nicht nur vollständig wieder ausgeglichen, das Papier konnte sogar auf ein neues Jahreshoch... ► Artikel lesen | |
| SOLID BIOSCIENCES | 6,750 | +20,43 % | Solid Biosciences Inc. - 8-K, Current Report | ||
| CG ONCOLOGY | 62,02 | +2,85 % | H.C. Wainwright Raises its Price Target on CG Oncology, Inc. (CGON) to $80 and Maintains a Buy Rating | ||
| AMYLYX PHARMACEUTICALS | 13,850 | -1,77 % | Amylyx Pharmaceuticals, Inc. - S-8, Securities to be offered to employees in employee benefit plans | ||
| APOGEE THERAPEUTICS | 73,08 | +2,99 % | RBC Capital lowers Apogee Therapeutics stock price target on dosing outlook | ||
| EVOTEC | 5,402 | -1,13 % | Shortseller-Positionen aktuell: Evotec, Gerresheimer, HelloFresh, Renk, Hypoport, Symrise, TUI | Wer Aktien leerverkauft, also auf fallende Kurse spekuliert (Shortselling), unterliegt bestimmten Transparenzpflichten. Aktuelle Meldungen über Leerverkäufe geben Aufschluss darüber. Diese Vorgaben... ► Artikel lesen | |
| TAYSHA GENE THERAPIES | 4,570 | +1,78 % | Taysha Gene Therapies, Inc.: Taysha Gene Therapies Announces Inducement Grant Under Nasdaq Listing Rule 5635(c)(4) | ||
| MODERNA | 45,130 | -0,29 % | Moderna freut sich über eine Zulassungsempfehlung der EMA für einen Kombi-Impfstoff gegen Corona und Grippe | Bei Corona-Impfstoffen war der US-Konzern Moderna noch ein Nachzügler. Anders scheint es nun bei Kombi-Impfstoffen zu laufen, die gleichzeitig vor Corona und der Grippe schützen. Dafür gibt es bisher... ► Artikel lesen |
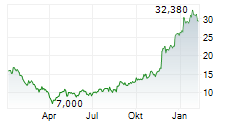
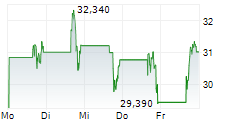
Das Unternehmen TYRA BIOSCIENCES INC kann der Branche Biotechnologie zugeordnet werden. Die relative Kursveränderung beträgt aktuell +1,51 % bei einem Aktienkurs von 35,070 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu TYRA BIOSCIENCES INC finden Sie auf Wallstreet Online.