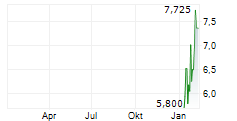| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
| 6,750 | 6,900 | 27.02. |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 20.02. | INSILICO (03696): VOLUNTARY ANNOUNCEMENT INCLUSION AS A CONSTITUENT OF HANG SENG COMPOSITE INDEX | 3 | HKEx | ||
| INSILICO MEDICINE Aktie jetzt für 0€ handeln | |||||
| 11.02. | Insilico and CMS launch drug discovery collaborations for CNS diseases | 3 | Pharmaceutical Technology | ||
| 10.02. | INSILICO (03696): VOLUNTARY ANNOUNCEMENT INSILICO MEDICINE AND CMS ENTER INTO DRUG DEVELOPMENT COLLABORATION VALUED AT SEVERAL TENS OF MILLIONS OF HKD ... | 2 | HKEx | ||
| 06.02. | Insilico Medicine appoints new VP of clinical development for oncology | 1 | Investing.com | ||
| 06.02. | Insilico Medicine Appoints Halle Zhang, PhD (Med), as Vice President, Clinical Development - Oncology | 159 | PR Newswire | CAMBRIDGE, Mass., Feb. 6, 2026 /PRNewswire/ -- Insilico Medicine ("Insilico") (3696.HK), a clinical-stage biotechnology company powered by generative artificial... ► Artikel lesen | |
| 05.02. | Insilico Medicine Nominates ISM5059, the Peripheral-restricted NLRP3 Inhibitor as Preclinical Candidate, for the Treatment of Multiple Inflammatory and Metabolism Indications | 90 | PR Newswire | NLRP3 is a validated anti-inflammatory target that mediates the release of proinflammatory cytokines IL-1ß and IL-18, and ISM5059 targets systemic inflammatory... ► Artikel lesen | |
| 03.02. | Listed IPO INSILICO Spikes 13% to Log New Listing High on $39M Milestone Payment Received Following First-in-patient Dosing for MEN2501 | 2 | AASTOCKS | ||
| 03.02. | INSILICO (03696): VOLUNTARY ANNOUNCEMENT INSILICO MEDICINE RECEIVES HKD39 MILLION MILESTONE PAYMENT FROM MENARINI GROUP FOLLOWING FIRST-IN-HUMAN ACHIEVEMENT ... | 1 | HKEx | ||
| 28.01. | Insilico and Qilu Pharmaceutical sign deal for cardiometabolic therapies | 1 | Pharmaceutical Technology | ||
| 27.01. | Insilico Medicine and Qilu Pharmaceutical Reach Near $120 Million Drug Development Collaboration to Accelerate Novel Cardiometabolic Therapies | 476 | PR Newswire | CAMBRIDGE, Mass., Jan. 27, 2026 /PRNewswire/ -- Insilico Medicine, a clinical-stage biotechnology company powered by generative AI, and Qilu Pharmaceutical Group... ► Artikel lesen | |
| 27.01. | Qilu programs path into cardiometabolic disease with $120M, AI-enabled Insilico alliance | 2 | FierceBiotech | ||
| 27.01. | INSILICO (03696): VOLUNTARY ANNOUNCEMENT INSILICO MEDICINE AND QILU PHARMACEUTICAL ENTER INTO DRUG DEVELOPMENT COLLABORATION VALUED AT OVER HKD931 MILLION ... | 2 | HKEx | ||
| 23.01. | Insilico Medicine Receives IND Approval from FDA for ISM8969, an AI-empowered Potential Best-in-class NLRP3 Inhibitor | 242 | PR Newswire | Investigational New Drug (IND) approval from the U.S. Food and Drug Administration (FDA) paves the way for ISM8969 clinical study in the United States. The Phase... ► Artikel lesen | |
| 23.01. | INSILICO (03696): VOLUNTARY ANNOUNCEMENT INSILICO MEDICINE RECEIVES IND APPROVAL FROM FDA FOR ISM8969, AN AI-EMPOWERED POTENTIAL BEST-IN-CLASS NLRP3 INHIBITOR | 2 | HKEx | ||
| 22.01. | INSILICO (03696): STABILIZING ACTIONS AND END OF STABILIZATION PERIOD | 1 | HKEx | ||
| 21.01. | Insilico agrees on $66M deal to split rights for preclinical NLRP3 inhibitor with new Chinese biotech | 7 | FierceBiotech | ||
| 21.01. | INSILICO (03696): NEXT DAY DISCLOSURE RETURN | - | HKEx | ||
| 21.01. | Insilico, Hygtia Therapeutics Partner To Advance ISM8969 NLRP3 Inhibitor For CNS Disorders | 1 | RTTNews | ||
| 21.01. | Insilico Medicine and Hygtia Therapeutics Enter New Global Strategic Collaboration to Co-Develop Novel Brain Penetrant NLRP3 Inhibitor for CNS Diseases treatment | 395 | PR Newswire | Insilico Medicine and Hygtia Therapeutics, an incubatee of Shenzhen Pengfu Fund of Fosun Health Capital and Fosun Pharma, have entered into an exclusive license... ► Artikel lesen | |
| 20.01. | INSILICO (03696): VOLUNTARY ANNOUNCEMENT INSILICO MEDICINE AND HYGTIA THERAPEUTICS ENTER INTO GLOBAL STRATEGIC COLLABORATION VALUED AT OVER HKD500 MILLION ... | 1 | HKEx |
1 2 3 Weiter >>
44 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| FRESENIUS | 50,90 | -0,82 % | DAX-Check: BMW, Fresenius, Hochtief, MTU, Vonovia, Zalando | Donald Trump wirbelt die Märkte mal wieder durcheinander. Der US-Präsident kündigte neue globale Zölle an. Zuvor hatte der Supreme Court die bisherigen Zollmaßnahmen für unrechtmäßig erklärt. Die Unsicherheit... ► Artikel lesen | |
| SIEMENS HEALTHINEERS | 42,140 | -0,59 % | RBC stuft Siemens Healthineers auf 'Outperform' | NEW YORK (dpa-AFX Analyser) - Die kanadische Bank RBC hat Siemens Healthineers mit einem Kursziel von 55 Euro auf "Outperform" belassen. Die Nervosität der Anleger vor einem möglichen Verkauf des Diagnostikgeschäfts... ► Artikel lesen | |
| FRESENIUS MEDICAL CARE | 39,450 | +0,59 % | GOLDMAN SACHS stuft FMC FRESENIUS MEDICAL CARE AG auf 'Neutral' | NEW YORK (dpa-AFX Analyser) - Die US-Investmentbank Goldman Sachs hat die Einstufung für FMC mit einem Kursziel von 40 Euro auf "Neutral" belassen. Das Ende des Jahres 2025 sei stark gewesen, schrieb... ► Artikel lesen | |
| ALIGNMENT HEALTHCARE | 19,220 | -5,83 % | Alignment Healthcare Reports Fourth Quarter and Full-Year 2025 Results; Beats High-End of Guidance Across All Key Metrics | Delivers full-year revenue of $3.95 billion, representing 46.1% growth year-over-year Exceeds high-end of fourth quarter and full-year guidance across all key metrics: membership, revenue, adjusted... ► Artikel lesen | |
| GERRESHEIMER | 16,950 | +7,96 % | SDAX-Wert im Bafin-Fokus: Gerresheimer stürzt immer tiefer | Der Druck auf den Düsseldorfer Verpackungsspezialisten steigt: Die Bundesanstalt für Finanzdienstleistungsaufsicht BaFin hat angekündigt, eine bereits laufende Bilanzkontrolle bei Gerresheimer auszuweiten... ► Artikel lesen | |
| BRIGHTSPRING HEALTH SERVICES | 41,380 | +3,14 % | BrightSpring Health Services, Inc. Reports Fourth Quarter and Full Year 2025 Financial Results and Provides Full Year 2026 Guidance | LOUISVILLE, Ky., Feb. 27, 2026 (GLOBE NEWSWIRE) -- BrightSpring Health Services, Inc. ("BrightSpring" or the "Company") (NASDAQ: BTSG), a leading provider of home and community-based health services... ► Artikel lesen | |
| CARL ZEISS MEDITEC | 27,120 | -0,59 % | Carl Zeiss Meditec AG: ZEISS launches Collaborative Care application to strengthen continuity of care; new solution offers on-premises and cloud-based options | Built on the ZEISS Health Data Platform, ZEISS Collaborative Care offers eye care professionals a flexible way to collaborate - whether as a standalone cloud application or as an integrated... ► Artikel lesen | |
| PROCEPT BIOROBOTICS | 22,670 | -4,10 % | PROCEPT BioRobotics Reports Fourth Quarter 2025 Financial Results and Updates 2026 Revenue Guidance | SAN JOSE, Calif., Feb. 25, 2026 (GLOBE NEWSWIRE) -- PROCEPT BioRobotics® Corporation (Nasdaq: PRCT) (the "Company"), a surgical robotics company focused on advancing patient care by developing transformative... ► Artikel lesen | |
| OTTOBOCK | 57,00 | +1,97 % | Ottobock sucht Käufer für einstiges Stammwerk in Thüringen | DUDERSTADT/KÖNIGSEE (dpa-AFX) - Der Prothesenhersteller Ottobock will sich von seiner Rollstuhlsparte am Standort Königsee in Thüringen trennen. Das Unternehmen aus Südniedersachsen sei mit mehreren... ► Artikel lesen | |
| ECKERT & ZIEGLER | 15,420 | +1,65 % | EQS-AFR: Eckert & Ziegler SE: Vorabbekanntmachung über die Veröffentlichung von Finanzberichten gemäß §§ 114, 115, 117 WpHG | EQS Vorabbekanntmachung Finanzberichte: Eckert & Ziegler SE
/ Vorabbekanntmachung über die Veröffentlichung von Rechnungslegungsberichten
Eckert & Ziegler SE: Vorabbekanntmachung... ► Artikel lesen | |
| PROGYNY | 17,685 | -20,55 % | Progyny, Inc. - S-8, Securities to be offered to employees in employee benefit plans | ||
| GENEDX | 79,77 | -1,87 % | These Analysts Slash Their Forecasts On GeneDx After Q4 Results | ||
| ONEMEDNET | 1,030 | +53,27 % | OneMedNet-Aktie steigt nach Plattform-Launch um 90 % | ||
| ESSILORLUXOTTICA | 225,00 | 0,00 % | FedEx, EssilorLuxottica sued by customers for tariff refunds | ||
| PRIVIA HEALTH GROUP | 23,730 | -4,81 % | Privia Health Group, Inc.: Privia Health Reports Fourth Quarter and Full-Year 2025 Financial Results | All 2025 Operating and Financial Metrics At or Above High End of Guidance RangesFull-year 2025 Net Income +59.3% from 2024Full-year 2025 Adjusted EBITDA of $125.5 Million, +38.8% from 2024Full-year... ► Artikel lesen |
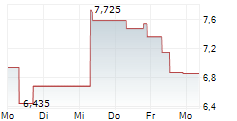
INSILICO MEDICINE CAYMAN TOPCO gehört der Branche Gesundheitswesen an. Der aktuelle Kurs liegt bei 6,900 Euro und damit +0,73 % im Plus.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu INSILICO MEDICINE CAYMAN TOPCO finden Sie auf Wallstreet Online.