- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Mi | Longeveron publishes phase 2b stem cell trial results in journal | 1 | Investing.com | ||
| LONGEVERON Aktie jetzt für 0€ handeln | |||||
| Mi | Longeveron Results of Phase 2b Clinical Trial Demonstrating Stem Cell Therapy Improved Condition of Patients with Age-Related Frailty Published in Cell Stem Cell | 292 | GlobeNewswire (Europe) | MIAMI, Feb. 25, 2026 (GLOBE NEWSWIRE) -- Longeveron Inc. (NASDAQ: LGVN), a clinical stage biotechnology company developing regenerative cell therapy for life-threatening rare pediatric and chronic... ► Artikel lesen | |
| 13.02. | Longeveron ernennt Stephen Willard vor entscheidenden Studienergebnissen zum neuen CEO | 1 | Investing.com Deutsch | ||
| 13.02. | Longeveron appoints Stephen Willard as new CEO ahead of pivotal trial results | 2 | Investing.com | ||
| 13.02. | Longeveron Inc. - 8-K, Current Report | 1 | SEC Filings | ||
| 13.02. | Longeveron Appoints Stephen H. Willard as Chief Executive Officer | 191 | GlobeNewswire (Europe) | Mr. Willard has a 30+ year track record of leadership across public and private sectors as CEO of multiple biotechnology and pharmaceutical firms, with an impressive history of delivering significant... ► Artikel lesen | |
| 13.02. | Longeveron appoints new CEO | 1 | Seeking Alpha | ||
| 05.02. | Longeveron Applauds Passage of the Mikaela Naylon Give Kids a Chance Act and Reauthorization of Rare Pediatric Disease Priority Review Voucher Program | 1 | GlobeNewswire (USA) | ||
| 29.01. | Longeveron Granted Japan Patent for Potency Assay Methods for Assessing Human Mesenchymal Stem Cells (MSCs) | 1 | GlobeNewswire (USA) | ||
| 26.01. | Longeveron prepares for pivotal HLHS therapy data with FDA meeting | 1 | Investing.com | ||
| 26.01. | Longeveron Announces FDA Grants Type C Meeting Ahead of Data Readout for Pivotal Phase 2 Clinical Trial (ELPIS II) Evaluating Treatment for Hypoplastic Left Heart Syndrome (HLHS) | 250 | GlobeNewswire (Europe) | ELPIS II top-line trial results are anticipated in the third quarter of 2026Laromestrocel Biological License Application (BLA) submission for full traditional approval for HLHS anticipated if ELPIS... ► Artikel lesen | |
| 29.12.25 | Longeveron, Selected as a StartUp Health Alzheimer's Disease Moonshot Company, Will Participate in StartUp Health Apollo House During JPM Healthcare Week 2026 | 5 | GlobeNewswire (USA) | ||
| 17.12.25 | Longeveron receives patent for stem cell therapy targeting female sexual dysfunction | 3 | Investing.com | ||
| 17.12.25 | Longeveron Granted U.S. Patent for Method of Treating Female Sexual Dysfunction Using its Proprietary Stem Cell Therapy | 1 | GlobeNewswire (USA) | ||
| 03.12.25 | Longeveron executives to speak at CVCT Forum on cell therapy trials | 1 | Investing.com | ||
| 03.12.25 | Longeveron: Führungskräfte präsentieren auf Fachforum für Zelltherapie-Studien | 1 | Investing.com Deutsch | ||
| 03.12.25 | Longeveron Chief Science Officer and Chief Medical Officer Selected as Speakers at the Global CardioVascular Clinical Trialists (CVCT) Forum | 1 | GlobeNewswire (USA) | ||
| 02.12.25 | Longeveron erhält kanadisches Patent für Stammzelltherapie | 1 | Investing.com Deutsch | ||
| 02.12.25 | Longeveron Granted Canadian Patent for Method of Using Stem Cells to Treat Non-Ischemic Dilated Cardiomyopathy and Aging-related Frailty in Patients with Inflammaging | 2 | GlobeNewswire (USA) | ||
| 01.12.25 | Longeveron's stem cell therapy shows reduced neuroinflammation in AD trial | 3 | Investing.com |
1 2 3 Weiter >>
47 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| CRISPR THERAPEUTICS | 50,50 | -3,81 % | Crispr Therapeutics gains amid takeover speculation | ||
| OCUGEN | 1,524 | -3,61 % | Ocugen Appoints Rita Johnson-Greene to Chief Financial Officer | MALVERN, Pa., Feb. 09, 2026 (GLOBE NEWSWIRE) -- Ocugen, Inc. (Ocugen or the Company) (NASDAQ: OCGN), a pioneering biotechnology leader in gene therapies for blindness diseases, today announced the... ► Artikel lesen | |
| EDITAS MEDICINE | 1,835 | -0,43 % | Editas Medicine, Inc.: Editas Medicine Nominates EDIT-401, an LDLR-Targeted Medicine, as Lead In Vivo Development Candidate | EDIT-401 achieved ~90% mean LDL-C reduction with single dose in non-human primates EDIT-401 on track for human proof-of-concept data by end of 2026 Strong cash position with operational runway into... ► Artikel lesen | |
| PACIFIC BIOSCIENCES OF CALIFORNIA | 1,438 | -4,91 % | PACIFIC BIOSCIENCES OF CALIFORNIA, INC. - S-8, Securities to be offered to employees in employee benefit plans | ||
| DENALI THERAPEUTICS | 17,705 | -2,51 % | Denali Therapeutics Inc.: Denali Therapeutics Reports Fourth Quarter and Full Year 2025 Financial Results and Business Highlights | Tividenofusp alfa (DNL310; ETV:IDS) launch readiness established ahead of April 5, 2026 Prescription Drug User Fee Act (PDUFA) target action date for Hunter syndromeDNL126 (ETV:SGSH) Phase 1/2 preliminary... ► Artikel lesen | |
| SOL GLOBAL INVESTMENTS | 0,058 | +5,50 % | SOL Global Investments Corp.: SOL Global Announces Leadership Transition and Grant of PSUs | Toronto, Ontario--(Newsfile Corp. - February 23, 2026) - SOL Global Investments Corp. (CSE: SOL) (FSE: 9SB) ("SOL Global" or the "Company"), today announced that Mr. Davide Marcotti has resigned... ► Artikel lesen | |
| TRINITY BIOTECH | 0,705 | -2,23 % | Trinity Biotech plc: Trinity Biotech Secures $25 Million Financing Commitment to Support Growth Initiatives | - Agreement provides additional funding tool of up to $25 million over a period of up to 36 months - Underpins the Company's ambitions to expand its commercialisation activities and advance its innovation... ► Artikel lesen | |
| FATE THERAPEUTICS | 1,247 | -6,56 % | Fate Therapeutics, Inc.: Fate Therapeutics Reports Fourth Quarter and Full Year 2025 Financial Results and Business Updates | Outpatient treatment enabled under FT819-102 autoimmune basket protocol with patients now treated with FT819 off-the-shelf CAR T-cell therapy as same-day hospital discharge Four systemic sclerosis... ► Artikel lesen | |
| VOYAGER THERAPEUTICS | 3,426 | -4,30 % | Voyager Therapeutics, Inc.: Voyager Reports Third Quarter 2025 Financial and Operating Results | - Momentum building around tau: expect VY7523 clinical data and VY1706 clinical entry in 2026 - - Sharpened focus on multi-modality pipeline with introduction of Voyager NeuroShuttle discovery... ► Artikel lesen | |
| ANAVEX LIFE SCIENCES | 3,701 | -1,96 % | Anavex Life Sciences Corp.: Anavex Life Sciences Appoints Seasoned Healthcare Leader to Board of Directors | NEW YORK, Feb. 23, 2026 (GLOBE NEWSWIRE) -- Anavex Life Sciences Corp. ("Anavex" or the "Company") (Nasdaq: AVXL), a clinical-stage biopharmaceutical company focused on developing innovative treatments... ► Artikel lesen | |
| ARCTURUS THERAPEUTICS | 8,240 | -0,96 % | Arcturus Therapeutics Provides Interim Phase 2 Data for Cystic Fibrosis Program | ARCT-032 generally safe and well tolerated
Meaningful trends of clinical activity observed via high resolution CT scans
After only 28 days of treatment with ARCT-032 (10 mg), 4 out of 6 Class... ► Artikel lesen | |
| BIOAGE LABS | 18,800 | -0,53 % | Oppenheimer initiates BioAge Labs stock coverage with outperform rating | ||
| CERUS | 2,210 | +0,09 % | Earnings Preview For Cerus | ||
| LINEAGE CELL THERAPEUTICS | 1,600 | -1,23 % | Regenerative Medizin im Aufbruch: NurExone Biologic, Regenxbio und Lineage Cell Therapeutics setzen auf unterschiedliche Zukunftstechnologien | ||
| OVID THERAPEUTICS | 1,390 | -1,42 % | Ovid Therapeutics Inc.: Ovid Therapeutics Appoints Dr. Petra Kaufmann as Chief Medical Officer | NEW YORK, Dec. 02, 2025 (GLOBE NEWSWIRE) -- Ovid Therapeutics Inc. (Nasdaq: OVID), a biopharmaceutical company developing small molecule medicines for brain disorders with significant unmet need,... ► Artikel lesen |
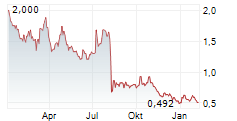
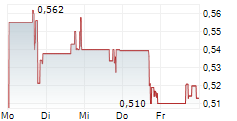
Das Unternehmen LONGEVERON INC kann der Branche Biotechnologie zugeordnet werden. Die relative Kursveränderung beträgt aktuell -7,26 % bei einem Aktienkurs von 0,541 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu LONGEVERON INC finden Sie auf Wallstreet Online.