- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 23.01. | REVIVA PHARMACEUTICALS HOLDINGS, INC. - 8-K, Current Report | 12 | SEC Filings | ||
| 05.01. | Chardan Capital assumes coverage on Reviva Pharmaceuticals stock with Buy rating | 11 | Investing.com | ||
| 29.12.25 | Reviva Pharmaceuticals: Reviva to Present at the Sachs 9th Annual Neuroscience Innovation Forum | 4 | GlobeNewswire (USA) | ||
| REVIVA PHARMACEUTICALS Aktie jetzt für 0€ handeln | |||||
| 24.12.25 | H.C. Wainwright bestätigt Kaufempfehlung für Reviva, senkt aber Kursziel nach FDA-Feedback | 17 | Investing.com Deutsch | ||
| 24.12.25 | Reviva Pharmaceuticals stock rating reiterated at Buy by H.C. Wainwright | 4 | Investing.com | ||
| 23.12.25 | FDA fordert weitere Studie: Aktie von Reviva Pharmaceuticals bricht ein | 36 | Investing.com Deutsch | ||
| 23.12.25 | FDA Asks Reviva Pharmaceuticals To Conduct Additional Phase 3 Study For Its Schizophrenia Drug | 4 | Benzinga.com | ||
| 23.12.25 | FDA fordert zweite Phase-3-Studie für Schizophrenie-Medikament von Reviva | 5 | Investing.com Deutsch | ||
| 23.12.25 | FDA recommends second phase 3 trial for Reviva's schizophrenia drug | 3 | Investing.com | ||
| 23.12.25 | Reviva Pharmaceuticals: Reviva Announces Regulatory Update Regarding the Development of Brilaroxazine for the Treatment of Schizophrenia | 473 | GlobeNewswire (Europe) | Written feedback from FDA pre-NDA meeting includes a recommendation to conduct a second Phase 3 trial to generate additional efficacy and safety data prior to NDA submission of brilaroxazine for the... ► Artikel lesen | |
| 23.12.25 | REVIVA PHARMACEUTICALS HOLDINGS, INC. - 8-K, Current Report | 1 | SEC Filings | ||
| 12.12.25 | NSE - REVIVA PHARMACEUTICALS HOLDINGS, INC. - 25, Notification of the removal from listing and registration of matured, redeemed or retired securities | 6 | SEC Filings | ||
| 13.11.25 | Reviva Pharmaceuticals: Reviva Reports Third Quarter 2025 Financial Results and Recent Business Highlights | 1.157 | GlobeNewswire (Europe) | - Pre-NDA meeting with FDA to discuss brilaroxazine's path to approval for schizophrenia planned in Q4 2025 - - Potential NDA submission for schizophrenia indication targeted for Q2 2026 - - European... ► Artikel lesen | |
| 13.11.25 | REVIVA PHARMACEUTICALS HOLDINGS, INC. - 8-K, Current Report | 4 | SEC Filings | ||
| 13.11.25 | REVIVA PHARMACEUTICALS HOLDINGS, INC. - 10-Q, Quarterly Report | - | SEC Filings | ||
| 28.10.25 | Reviva to present brilaroxazine schizophrenia data at CNS Summit | 4 | Investing.com | ||
| 28.10.25 | Reviva Pharmaceuticals: Reviva to Present Negative Symptom Data for Brilaroxazine in Schizophrenia from the Phase 3 RECOVER Double-Blind and Open-Label Extension Trials at the CNS Summit 2025 | 4 | GlobeNewswire (USA) | ||
| 27.10.25 | H.C. Wainwright slashes Reviva Pharmaceuticals stock price target on dilution concerns | 7 | Investing.com | ||
| 17.10.25 | REVIVA PHARMACEUTICALS HOLDINGS, INC. - 8-K, Current Report | 6 | SEC Filings | ||
| 26.09.25 | Reviva Pharmaceuticals senkt Präsenzquorum für Hauptversammlungen | 18 | Investing.com Deutsch |
1 2 Weiter >>
33 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BAYER | 41,325 | -1,24 % | Bayer: Kaufen, halten oder verkaufen - wie wirkt der Chart? | Bayer konsolidiert nach einer starken Aufwärtsphase derzeit im Bereich der 50-Tage-Linie. Hält diese Zone und entsteht daraus ein erneuter Impuls nach oben Den vollständigen Artikel lesen ... ► Artikel lesen | |
| NOVO NORDISK | 31,605 | -0,22 % | Novo Nordisk: Nächster Nackenschlag | Im aufstrebenden Adipositas-Markt droht der dänische Biopharma-Riese Novo Nordisk weiter hinter den US-Wettbewerber Eli Lilly zurückzufallen. Neue Daten zum Hoffnungsträger CagriSema sorgen erneut für... ► Artikel lesen | |
| ROCHE | 397,55 | -1,50 % | Hightech-Neubau für frühe Krankheitsdiagnosen: Drees & Sommer begleitete Roche bei Millionenprojekt | Penzberg (ots) - Im neu eingeweihten Diagnostik- und Innovationszentrum in Penzberg werden in Zukunft In-vitro-Diagnostika und spezifische Testverfahren entwickelt, um Krankheiten früh zu erkennen.Alzheimer... ► Artikel lesen | |
| VIATRIS | 12,485 | -1,03 % | Viatris Posts Narrower Loss In Q4; Expects Workforce Reduction Of Up To Approx. 10% | WASHINGTON (dpa-AFX) - Viatris Inc. (VTRS) reported a fourth quarter net loss of $340.1 million or $0.30 per share compared to a loss of $516.5 million or $0.43 per share, prior year. The company... ► Artikel lesen | |
| GREEN THUMB INDUSTRIES | 5,610 | +1,08 % | Green Thumb Industries Reports Fourth Quarter and Full Year 2025 Results | CHICAGO and VANCOUVER, British Columbia, Feb. 25, 2026 (GLOBE NEWSWIRE) -- Green Thumb Industries Inc. ("Green Thumb" or the "Company") (CSE: GTII) (OTCQX: GTBIF), a leading national cannabis consumer... ► Artikel lesen | |
| TONIX PHARMACEUTICALS | 11,700 | -1,68 % | Tonix Pharmaceuticals Holding Corp.: Tonix Pharmaceuticals Announces Program Updates on Phase 2/3-Ready Long-Acting Monoclonal Antibody (mAb) Designed for Seasonal Prevention of Lyme Disease (TNX-4800) | Exploring clinical development plan options including a controlled human infection model (CHIM) and a Phase 2/3 adaptive field study Expect to have investigational product of TNX-4800 (anti-Borrelia... ► Artikel lesen | |
| NEWRON PHARMACEUTICALS | 20,450 | -3,08 % | NEWRON PHARMACEUTICALS SPA: Stabilität als Signal | ||
| CASSAVA SCIENCES | 1,923 | -0,54 % | Cassava Sciences, Inc.: Cassava Announces Agreement to Settle Securities Class Action Litigation | AUSTIN, Texas, Dec. 23, 2025 (GLOBE NEWSWIRE) -- Cassava Sciences, Inc. (NASDAQ: SAVA, "Cassava", the "Company"), a biotechnology company focused on developing novel, investigational treatments for... ► Artikel lesen | |
| ORAMED PHARMACEUTICALS | 2,858 | -0,04 % | Dividendenbekanntmachungen (16.01.2026) | Unternehmen ISIN-Code Dividende (Währung) Dividende (EUR) ABBVIE INC US00287Y1091 1,73 USD 1,4902 EUR ABBVIE INC CDR CA00288K1084 0,2439 CAD 0,1512 EUR ALAMO GROUP INC US0113111076 0... ► Artikel lesen | |
| COMPASS PATHWAYS | 5,950 | +1,71 % | Compass Pathfinder Limited: Compass Pathways Announces Exercise of $200 Million in Outstanding Warrants | Compass Pathways plc (Nasdaq: CMPS), a biotechnology company dedicated to accelerating patient access to evidence-based innovation, announced today the exercise of 35,059,448 warrants, which were... ► Artikel lesen | |
| ACHIEVE LIFE SCIENCES | 3,825 | -1,16 % | Achieve Life Sciences: Entscheidet der 20. Juni über Leben und Tod? | Seit unseres Trading-Tipps im Juli letzten Jahres konnte die Aktie von Achieve Life Sciences zwischenzeitlich über +150% zulegen, korrigierte in den letzten Wochen aber wieder um mehr als 20%. Das Unternehmen... ► Artikel lesen | |
| OPUS GENETICS | 3,180 | -9,66 % | Opus Genetics, Inc.: Opus Genetics Announces Initial Clinical Data from Phase 1/2 OPGx-BEST1 Gene Therapy Study at the Macula Society Annual Meeting | Sentinel participant showed OPGx-BEST1 was well tolerated with no ocular inflammation, treatment-related adverse events, or dose-limiting toxicities at three months Early signals of functional and... ► Artikel lesen | |
| AURINIA PHARMACEUTICALS | 11,820 | -1,46 % | Aurinia targets $305M-$315M LUPKYNIS sales for 2026 while advancing aritinercept clinical development | ||
| KAZIA THERAPEUTICS | 8,430 | -4,15 % | Kazia Therapeutics Limited: Kazia Therapeutics Announces Compelling Preclinical and Translational Data for Nuclear PD-L1 Degrader (NDL2) | Data identify nuclear PD-L1 as a previously unaddressed driver of immunotherapy resistance and metastatic progression
SYDNEY, Jan. 30, 2026 /PRNewswire/ -- Kazia... ► Artikel lesen | |
| MINDWALK | 0,930 | -3,63 % | MindWalk setzt HYFT-Technologie zur Erkennung funktionaler Adjazenz mit Bezug zu Portfoliorisiken bei KI-designten Therapeutika ein | AUSTIN, Texas / IRW-Press / MindWalk Holdings Corp. (NASDAQ:HYFT) ("MindWalk" oder das "Unternehmen"), ein Bio-Native-AI-Unternehmen für therapeutische
Forschung und Technologie, gab heute die... ► Artikel lesen |
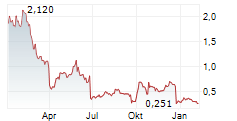
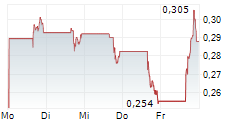
REVIVA PHARMACEUTICALS HOLDINGS INC gehört der Branche Pharma an. Der aktuelle Kurs liegt bei 0,218 Euro und damit 0,00 % im Plus.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu REVIVA PHARMACEUTICALS HOLDINGS INC finden Sie auf Wallstreet Online.