| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
| 13,480 | 13,620 | 13:36 | |
| 13,465 | 13,495 | 13:28 |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| ASTELLAS PHARMA Aktie jetzt für 0€ handeln | |||||
| 08:13 | Zydus Lifesciences Finalizes Rs 120 Million Settlement With Astellas Over Generic Mirabegron Sales In US | 2 | The Free Press Journal | ||
| 06:26 | Zydus Lifesciences gains on settlement with Astellas over Myrbetriq | 7 | capitalmarket.com | ||
| Di | Lupin settles Myrbetriq patent spat with Astellas for $90m | 1 | pharmaphorum | ||
| Di | India's Lupin clears patent hurdle with Astellas Pharma, keeps Mirabegron sales in US | 5 | Reuters | ||
| 05.02. | Astellas tops expectations as Vyloy sales surge outshines trial setback | 6 | FiercePharma | ||
| 04.02. | Earnings Call Transkript: Astellas Pharma meldet Umsatzplus für Q3 2025, Aktie steigt | 6 | Investing.com Deutsch | ||
| 04.02. | Astellas Pharma ADR GAAP EPS of ¥185.20, revenue of ¥1601.32B; updates full-year forecast | 18 | Seeking Alpha | ||
| 15.01. | JPM26: Astellas CEO resists 'rescue BD' as $6B Xtandi patent cliff nears | 17 | FiercePharma | ||
| 15.01. | Ocugen posts midphase gene therapy data, charting course for rivalry with Apellis, Astellas | 37 | FierceBiotech | ||
| 08.01. | Pfizer, Astellas Report Positive Phase 3 Trial Results for PADCEV Combination in Bladder Cancer Treatment | 18 | Insider Monkey | ||
| 18.12.25 | Astellas Reports Positive Phase 3 EV-304 Results For PADCEV Combo In Muscle-Invasive Bladder Cancer | 500 | AFX News | NEW YORK CITY (dpa-AFX) - Astellas Pharma Inc. and Pfizer Inc. announced positive topline results from the Phase 3 EV-304 clinical trial (KEYNOTE-B15), evaluating PADCEV (enfortumab vedotin)... ► Artikel lesen | |
| 17.12.25 | Pfizer, Astellas post late-stage trial win for Padcev in bladder cancer with Merck's Keytruda | 10 | Seeking Alpha | ||
| 17.12.25 | Astellas Pharma Inc.: PADCEV Plus Keytruda Significantly Improves Survival for Patients with Muscle-Invasive Bladder Cancer Regardless of Cisplatin Eligibility | 347 | PR Newswire | PADCEV plus Keytruda is the first and only regimen without platinum-based chemotherapy to improve event-free and overall survival when used before and after surgery... ► Artikel lesen | |
| 15.12.25 | Aviceda geographic atrophy hopeful fails to top Astellas med in phase 2 | 5 | FierceBiotech | ||
| 01.12.25 | EMA Validates Review Of Astellas' PADCEV And KEYTRUDA Combination In Bladder Cancer | 771 | AFX News | TOKYO (dpa-AFX) - Astellas Pharma Inc. (ALPMY, 4503.T) announced that the European Medicines Agency (EMA) has validated for review a Type II variation application for PADCEV (enfortumab vedotin).... ► Artikel lesen | |
| 31.10.25 | Ajinomoto, Astellas Enter ADC Technology Agreement | 3 | Contract Pharma | ||
| 30.10.25 | Astellas Pharma gives H1 results | 18 | Seeking Alpha | ||
| 30.10.25 | Astellas Pharma H1 Net Profit Surges, Revenue Improves; Lifts Annual Outlook | 533 | AFX News | TOKYO (dpa-AFX) - Astellas Pharma Inc. (ALPMY, 4503.T), a Japanese pharmaceutical company, on Thursday reported a surge in net profit for the first half. In addition, the company has revised... ► Artikel lesen | |
| 27.10.25 | Bayer Menopause Drug Lands FDA Approval, Bringing Competition to an Astellas Product | 20 | MedCity News | ||
| 26.10.25 | Pfizer Inc. (PFE) and Astellas Pharma Announce Survival Results from Phase 3 EMBARK Study | 51 | Insider Monkey |
1 2 3 Weiter >>
51 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BAYER | 46,680 | +1,20 % | Bayer punktet mit Asundexian: Neue Studiendaten machen Hoffnung auf Milliarden-Umsätze | Bayer hat die ersehnten Studiendaten zu seiner Pharmahoffnung Asundexian präsentiert. Die Ergebnisse zeigen eine deutliche Reduktion von Schlaganfällen - Analysten sind optimistisch für das Umsatzpotenzial... ► Artikel lesen | |
| NOVO NORDISK | 41,205 | +0,38 % | Almonty Aktie geht durch die Decke! Kurshorror bei Novo Nordisk! Und was macht Hensoldt? | Über 17 % hat die Aktie von Almonty am Dienstag an der Nasdaq zulegen können. Der größte westliche Wolfram-Produzent dürfte von dem Aufbau einer strategischen Reserve bei kritischen Rohstoffen durch... ► Artikel lesen | |
| PFIZER | 23,290 | -0,26 % | FDA prüft Zulassungserweiterung für Pfizers Hämophilie-Medikament Hympavzi | Die US-Arzneimittelbehörde FDA hat dem Pharmakonzern Pfizer für das Hämophilie-Medikament Hympavzi eine vorrangige Prüfung einer beantragten Zulassungserweiterung eingeräumt. Der ergänzende Antrag betrifft... ► Artikel lesen | |
| NOVARTIS | 134,40 | -0,53 % | DEUTSCHE BANK RESEARCH stuft NOVARTIS AG auf 'Buy' | FRANKFURT (dpa-AFX Analyser) - Deutsche Bank Research hat die Einstufung für Novartis mit einem Kursziel von 125 Franken auf "Buy" belassen. Dies schrieb Emmanuel Papadakis in seiner am Donnerstag vorliegenden... ► Artikel lesen | |
| MERCK KGAA | 126,55 | +0,96 % | JPMORGAN stuft MERCK KGAA auf 'Overweight' | NEW YORK (dpa-AFX Analyser) - Die US-Bank JPMorgan hat die Einstufung für Merck KGaA mit einem Kursziel von 150 Euro auf "Overweight" belassen. Analyst Richard Vosser geht davon aus, dass der Ausblick... ► Artikel lesen | |
| GILEAD SCIENCES | 131,18 | -0,02 % | Gilead Sciences: Weitere Zukäufe geplant - diese Aktie könnte profitieren | Seit September hatten wir immer wieder auf ein bevorstehendes Ausbruchsszenario bei der Aktie des Pharmariesens Gilead Sciences verwiesen - und schlussendlich eindrucksvoll Recht behalten. Seit unseres... ► Artikel lesen | |
| AURORA CANNABIS | 2,885 | -0,69 % | Aurora Cannabis Inc.: Aurora Advances Global Medical Growth Strategy with Portfolio Expansion in Australia & New Zealand | NASDAQ| TSX: ACB
Launch reinforces Aurora's leadership position across key international markets
EDMONTON, AB, Feb. 11, 2026 /CNW/ - Aurora Cannabis Inc. (NASDAQ:... ► Artikel lesen | |
| SANOFI | 78,67 | -4,65 % | Sanofi holt Merck-Chefin Garijo als CEO | DJ Sanofi holt Merck-Chefin Garijo als CEO
Von Billy Gray
DOW JONES--Sanofi wechselt den CEO aus. Wie der französische Pharmakonzern mitteilte, hat er das Mandat von Paul Hudson nicht verlängert.... ► Artikel lesen | |
| GSK | 24,520 | -0,81 % | DEUTSCHE BANK RESEARCH stuft GSK auf 'Hold' | FRANKFURT (dpa-AFX Analyser) - Deutsche Bank Research hat die Einstufung für GSK mit einem Kursziel von 1675 Pence auf "Hold" belassen. Die Ergebnisse der Briten im viertel Quartal seien durchwachsen... ► Artikel lesen | |
| ABBVIE | 186,60 | +0,32 % | 1 Reason I'm Never Selling AbbVie Stock | ||
| CANOPY GROWTH | 0,913 | +2,13 % | Cannabis Frust: Zahlen bringen keine Wende für Canopy Growth | Der einstige Highflyer der Cannabis-Branche kommt einfach nicht vom Fleck. Zwar konnte Canopy Growth beim Umsatz überraschen, doch unter dem Strich steht erneut ein dickes Minus. Anleger reagieren ernüchtert... ► Artikel lesen | |
| ROCHE | 392,00 | +0,87 % | CH-Eröffnung: Knappes Minus - Gewinnmitnahmen bei Roche und Novartis | Zürich - Der Schweizer Aktienmarkt ist am Donnerstag kaum verändert in den Handel gestartet. Eine Mehrheit der Titel steht zwar im Plus, Gewinnmitnahmen bei den beiden Pharmaschwergewichten Roche und... ► Artikel lesen | |
| STADA ARZNEIMITTEL | - | - | Pharmakonzern Stada: Nachträglich mehr Geld für Aktionäre | Hartnäckigkeit kann sich für Anleger auszahlen. Zur Höhe der gezahlten Abfindung beim Stada-Squeeze-Out sind immer noch Klagen ehemaliger Aktionäre anhängig. So ist der aktuelle Stand. Der Hintergrund:... ► Artikel lesen | |
| ELI LILLY | 860,80 | +0,70 % | Novo Nordisk und Eli Lilly atmen auf - FDA schiebt Hims & Hers einen Riegel vor | Eli Lilly und Novo Nordisk haben ein Problem weniger und die Anleger werden wieder mutiger. Die US-Arzneimittelbehörde FDA verschärft den regulatorischen Druck auf den boomenden Markt für Abnehmmedikamente.... ► Artikel lesen | |
| MERCK & CO | 100,60 | 0,00 % | FDA Approves Merck's Keytruda And Keytruda Qlx For Platinum-Resistant Ovarian Cancer | KENILWORTH (NJ) (dpa-AFX) - Merck & Co Inc. (MRK) on Wednesday said the U.S. Food and Drug Administration has approved Keytruda and Keytruda Qlx, a subcutaneous formulation of Keytruda, in combination... ► Artikel lesen |
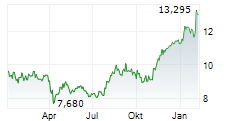
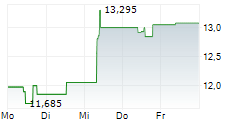
Das Unternehmen ASTELLAS PHARMA INC kann der Branche Pharma zugeordnet werden. Mit einer aktuellen Kursveränderung von -0,160 (-1,16 %) liegt der Kurs bei 13,600 Euro. Die Dividendendrendite beträgt aktuell +3,09 % (berechnet anhand der Ausschüttung von 0,42 Euro in den letzten 12 Monaten).
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu ASTELLAS PHARMA INC finden Sie auf Wallstreet Online.