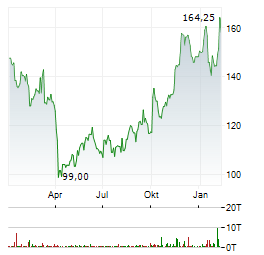
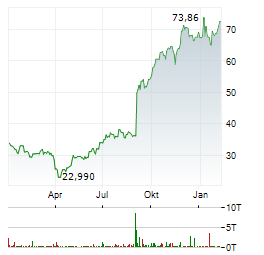
Chart-Typ:
Zeitraum:
| FDA Grants Fast Track Designation To J&J's Nipocalimab For Systemic Lupus Erythematosus | NEW BRUNSWICK (dpa-AFX) - Johnson & Johnson (JNJ) on Tuesday said its nipocalimab has been granted Fast Track designation by the U.S. Food and Drug Administration for the treatment of adults... ► Artikel lesen |
| Johnson & Johnson therapy nipocalimab granted U.S. FDA Fast Track designation in systemic lupus erythematosus (SLE) | Fast Track designation reflects the unmet need in this serious disease and enables the potential for an accelerated FDA review timeline
The designation is supported... ► Artikel lesen |
| Early study results from Johnson & Johnson show promising antitumor activity with combination of pasritamig and docetaxel in advanced prostate cancer | Combination demonstrates deep PSA responses and favorable safety profile with plans to advance into Phase 3
Data highlight the potential of this first-in-class next-generation... ► Artikel lesen |
| Biogen Inc.: The New England Journal of Medicine Publishes First Data to Demonstrate the Potential for Disease Modification in Dravet Syndrome | -Results show that targeting the underlying cause of Dravet syndrome with zorevunersen may improve outcomes for people with this rare, devastating genetic neurodevelopmental disease-
-Data support... ► Artikel lesen |
| WONBIOGEN Co., Ltd: Won BioGen Accelerates Global Market Expansion with Advanced Moist Wound Care Technology | SEOUL, South Korea, Feb. 24, 2026 /PRNewswire/ -- Medical device manufacturer Won BioGen (CEO Kim Won-il) is accelerating its global market expansion based on its advanced moist wound care... ► Artikel lesen |
| Biogen und BioArctic: Debatte um Alzheimer-Mittel in Deutschland spitzt sich zu | Im vergangenen Jahr hat Biogen mit seinem japanischen Partner Eisai das Alzheimer-Medikament Leqembi (Lecanemab) auch in Deutschland auf den Markt gebracht. Doch es herrschen Zweifel an der Wirksamkeit... ► Artikel lesen |
| EU Approves DAWNZERA For Hereditary Angioedema, Says Ionis And Otsuka | TOKYO (dpa-AFX) - Ionis Pharmaceuticals, Inc. (IONS) and Otsuka Pharmaceutical Co., Ltd. on Wednesday said the European Commission has approved Dawnzera to prevent repeated attacks of hereditary... ► Artikel lesen |
| Ionis Pharmaceuticals, Inc.: DAWNZERA (donidalorsen) approved in the European Union for hereditary angioedema (HAE) | Ionis Pharmaceuticals, Inc. (Nasdaq: IONS) and Otsuka Pharmaceutical Co., Ltd. (Otsuka) today announced that the European Commission (EC) has approved DAWNZERA (donidalorsen) in the European Union... ► Artikel lesen |
| Ionis Pharmaceuticals, Inc.: TRYNGOLZA (olezarsen) approved in the European Union for familial chylomicronemia syndrome (FCS) | Ionis Pharmaceuticals, Inc. (Nasdaq: IONS) and Sobi® today announced that TRYNGOLZA® (olezarsen) has been approved in the European Union (EU) as an adjunct to diet in adult patients for the treatment... ► Artikel lesen |
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu BG MEDICINE INC finden Sie auf Wallstreet Online.