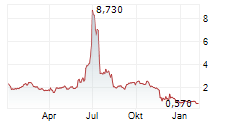
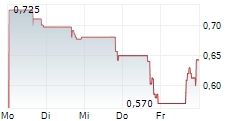
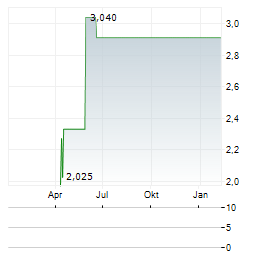
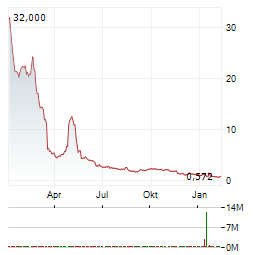
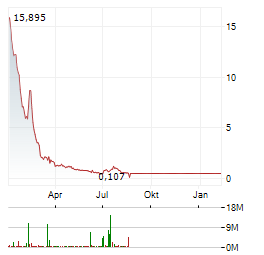
Chart-Typ:
Zeitraum:
| Sonoma Pharmaceuticals, Inc.: Sonoma Pharmaceuticals Reports Third Fiscal Quarter 2026 Financial Results | Revenue increased 22% for the quarter ended December 31, 2025 and 33% for the nine months ended December 31, 2025 compared to prior yearNet loss and net loss per share decreased from prior year for... ► Artikel lesen |
| Sonoma Pharmaceuticals, Inc.: Sonoma Pharmaceuticals Welcomes New Member of its Board of Directors and New Senior Vice President of Regulatory, Quality and Product Development | BOULDER, CO / ACCESS Newswire / January 28, 2026 / Sonoma Pharmaceuticals, Inc. (Nasdaq:SNOA), a global healthcare leader developing and producing patented Microcyn technology based stabilized hypochlorous... ► Artikel lesen |
| Sonoma Pharmaceuticals, Inc.: National Rosacea Society Awards Seal of Acceptance to Sonoma Pharmaceuticals for Reliefacyn Advanced | BOULDER, CO / ACCESS Newswire / November 13, 2025 / Sonoma Pharmaceuticals, Inc. (Nasdaq:SNOA), a global healthcare leader developing and producing patented Microcyn® technology based stabilized hypochlorous... ► Artikel lesen |
| Jaguar Health, Inc.: Jaguar Health Secures an Additional $3 Million Payment from Future Pak for Mytesi and Canalevia-CA1 | As announced, Jaguar received an up-front payment of $16M of non-dilutive capital in January 2026 upon entering U.S. license agreement with Future Pak for Mytesi and Canalevia-CA1 - Jaguar's two commercialized... ► Artikel lesen |
| Jaguar Health, Inc.: Reminder: Today, March 2, 2026, is the Record Date for Jaguar Health's Special One-time Stock Dividend | Dividend intended to provide dilution protection to Jaguar shareholders as company explores pathway to restructure debt SAN FRANCISCO, CALIFORNIA / ACCESS Newswire / March 2, 2026 / Jaguar Health, Inc.... ► Artikel lesen |
| XFRA CAPITAL ADJUSTMENT INFORMATION - 27.02.2026 | Das Instrument UZ9 CA4577022078 INSPIRATION ENERGY CORP. EQUITY wird cum Kapitalmassnahme gehandelt am 27.02.2026 und ex Kapitalmassnahme am 02.03.2026 The instrument UZ9 CA4577022078 INSPIRATION ENERGY... ► Artikel lesen |
| Windtree Therapeutics Announces It Has Signed a Letter of Intent to Acquire CommLoan, Inc. a Revenue Generating Company in the Rapidly Growing Fintech Space | Windtree is executing its corporate strategy to diversify its business with revenue and profit generating divisions CommLoan, Inc. is a tech company focused on transforming the way middle market loans... ► Artikel lesen |
| Grosvenor Invests in Wint to Strengthen Water Intelligence and Sustainability Across Global Real Estate | "Water risk and water waste are growing issues for the built environment, and Wint's technology will deliver clear value across our portfolio."BOSTON and LONDON, Dec. 3, 2025 /PRNewswire/... ► Artikel lesen |
| Windtree Therapeutics Announces It May Receive License Agreement Payments from Its Licensee for Renewed Acute Pulmonary Development by the Licensee | The Company has a global license agreement for the acute pulmonary that could pay up to $78.9 million in development, regulatory and commercial milestones plus low double digit royalties The licensed... ► Artikel lesen |
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu NETCAPITAL INC finden Sie auf Wallstreet Online.