- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| JAGUAR HEALTH Aktie jetzt für 0€ handeln | |||||
| Mo | Jaguar Health, Inc.: Reminder: Today, March 2, 2026, is the Record Date for Jaguar Health's Special One-time Stock Dividend | 165 | ACCESS Newswire | Dividend intended to provide dilution protection to Jaguar shareholders as company explores pathway to restructure debt SAN FRANCISCO, CALIFORNIA / ACCESS Newswire / March 2, 2026 / Jaguar Health, Inc.... ► Artikel lesen | |
| Fr | XFRA CAPITAL ADJUSTMENT INFORMATION - 27.02.2026 | 180 | Xetra Newsboard | Das Instrument UZ9 CA4577022078 INSPIRATION ENERGY CORP. EQUITY wird cum Kapitalmassnahme gehandelt am 27.02.2026 und ex Kapitalmassnahme am 02.03.2026 The instrument UZ9 CA4577022078 INSPIRATION ENERGY... ► Artikel lesen | |
| 18.02. | Jaguar Health, Inc.: Jaguar Health Announces a Special One-time Stock Dividend | 358 | ACCESS Newswire | Dividend intended to provide dilution protection to Jaguar shareholders as company explores pathway to restructure debt SAN FRANCISCO, CA / ACCESS Newswire / February 18, 2026 / Jaguar Health, Inc.... ► Artikel lesen | |
| 18.02. | Jaguar Health, Inc. - 8-K, Current Report | 3 | SEC Filings | ||
| 12.02. | Original-Research: Jaguar Health Inc (von First Berlin Equity Research GmbH): BUY | 232 | EQS Group (EN) | Original-Research: Jaguar Health Inc - from First Berlin Equity Research GmbH
12.02.2026 / 15:47 CET/CEST
Dissemination of a Research, transmitted by EQS News - a service of EQS Group.
The issuer... ► Artikel lesen | |
| 23.01. | Jaguar Health, Inc. - 8-K, Current Report | 3 | SEC Filings | ||
| 22.01. | Jaguar Health, Inc.: Jaguar Health Highlights Sharp Strategic Focus on Rare Intestinal Failure Diseases Fueled by Non-Dilutive Funds from Closing of License Deal for Mytesi | 583 | ACCESS Newswire | Jaguar has received the initial $16M payment related to the company's recently executed US out-license agreement for Mytesi and Canalevia-CA1, which has the potential to provide Jaguar up to an additional... ► Artikel lesen | |
| 14.01. | Jaguar Health, Inc.: Jaguar Health Presenting January 15 at Lytham Partners Healthcare Investor Summit to Provide Updates on Near-Term Catalysts | 425 | ACCESS Newswire | SAN FRANCISCO, CA / ACCESS Newswire / January 14, 2026 / Jaguar Health, Inc. (NASDAQ:JAGX) today announced that Lisa Conte, the company's founder, president and CEO, will present virtually on Thursday... ► Artikel lesen | |
| 12.01. | Jaguar Health, Inc.: Jaguar Health Enters into U.S. License Agreement with Future Pak for Crofelemer, Providing up to $38 Million | 488 | ACCESS Newswire | $18M upfront payment to Jaguar ($16M upon deal closing and $2M upon completion of post-closing conditions)Up to additional $20M in milestone and other future paymentsFuture Pak becomes exclusive U.S.... ► Artikel lesen | |
| 12.01. | Jaguar Health, Inc. - 8-K, Current Report | 8 | SEC Filings | ||
| 06.01. | Jaguar Health, Inc.: Article About Groundbreaking Results from Study of Jaguar Health's Crofelemer for Treatment of Intestinal Failure Featured in United Arab Emirates Healthcare Publication | 381 | ACCESS Newswire | Article discusses results demonstrating parenteral support (PS) reduction ranging from 12 to 37% in ongoing proof-of-concept study of crofelemer in pediatric patients with intestinal failureAssociated... ► Artikel lesen | |
| 02.01. | Jaguar Health, Inc.: Jaguar Health Awarded $240,000 FDA Grant in Support of Canalevia-CA1 for Treatment of Chemotherapy-Induced Diarrhea in Dogs | 513 | ACCESS Newswire | SAN FRANCISCO, CALIFORNIA / ACCESS Newswire / January 2, 2026 / Jaguar Health, Inc. (NASDAQ:JAGX) ("Jaguar") today announced that it received notice from the U.S. Food and Drug Administration's Center... ► Artikel lesen | |
| 15.12.25 | Jaguar Health, Inc.: Jaguar Health Announces Abstract Submission for Preliminary Data from US Investigator-Initiated Trial of Crofelemer in Adult Patients with Short Bowel Syndrome with Intestinal Failure | 353 | ACCESS Newswire | SBS-IF is the second orphan disease target indication for Jaguar's intestinal failure program, which includes microvillus inclusion disease (MVID) - for which the company completed a meeting in October... ► Artikel lesen | |
| 10.12.25 | FDA extends conditional approval for Jaguar Health's canine drug | 17 | Investing.com | ||
| 10.12.25 | Jaguar Health, Inc.: FDA Approves Renewal of Canalevia-CA1, Jaguar Health's Drug for Chemotherapy-Induced Diarrhea in Dogs | 334 | ACCESS Newswire | Conditional approval extended through December 2026 for the treatment of CID in dogsCID confirmatory effectiveness trial expected to conclude in February 2026, ahead of FDA's June deadline - 51 dogs... ► Artikel lesen | |
| 08.12.25 | Jaguar Health, Inc.: Jaguar Health Reports Approval of All Proposals at December 2025 Special Meeting of Stockholders | 482 | ACCESS Newswire | Jaguar CEO Lisa Conte presenting December 10 from 3:25pm - 3:35pm Eastern at the Emerging Growth Conference to provide updates on near-term catalysts; Click here to registerInitial results of ongoing... ► Artikel lesen | |
| 08.12.25 | Jaguar Health, Inc. - 8-K, Current Report | - | SEC Filings | ||
| 02.12.25 | Jaguar Health beantragt EMA-Beratung für EU-Zulassung von Tiermedikament | 14 | Investing.com Deutsch | ||
| 02.12.25 | Jaguar Health seeks EMA advice on EU approval for canine diarrhea drug | 2 | Investing.com | ||
| 02.12.25 | Jaguar Health, Inc.: Jaguar Health Makes Submission to EMA Regarding EU Approval Pathway for Canalevia for General Diarrhea in Dogs Based on Data from Completed Study | 1.817 | ACCESS Newswire | Jaguar's requesting advice from EMA on EU approval pathway for general diarrhea of FDA conditionally approved CanaleviaA novel non-antibiotic approach to diarrhea treatment is important because there... ► Artikel lesen |
1 2 3 4 5 Weiter >>
91 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BAYER | 40,610 | 0,00 % | Novo Nordisk Aktie: Schon wieder Kurssturz! - Bayer, Hometogo, Indus, Serviceware und TUI - Börse Frankfurt | Wo ist am Aktienmarkt etwas los, welche Themen interessieren Anleger derzeit besonders? Vor allem für Trader ist es wichtig zu wissen, wo "die Musik spielt" und welche Themen an der Börse aktuell besonders... ► Artikel lesen | |
| NOVO NORDISK | 32,005 | -0,51 % | maydornsmeinung: Silber, Nvidia, AMD, Microsoft, IBM, SAP, TeamViewer, Novo Nordisk, Nebius & Co. | In maydornsmeinung geht es zunächst um den Gesamtmarkt: Trotz Zoll-Chaos bleiben für Alfred die Unternehmensgewinne der entscheidende Faktor. Die Berichtssaison läuft stark, auch wenn sich die großen... ► Artikel lesen | |
| PFIZER | 23,280 | -0,11 % | Amt warnt vor Honigpaste mit verstecktem Viagra-Wirkstoff | TÜBINGEN (dpa-AFX) - Das Landratsamt Tübingen warnt vor dem Verzehr einer bei Amazon verkauften Honigpaste - weil ein verstecktes Potenzmittel drin ist. Das Nahrungsergänzungsmittel "Lotus Mixed Herbal... ► Artikel lesen | |
| NOVARTIS | 142,00 | -0,73 % | Novartis tritt in Indien den Rückzug an, um sich in Zukunft noch mehr auf das US-Geschäft konzentrieren zu können | Seit rund einem Jahr verstärkt Novartis seine Bemühungen um den US-Markt. Aus Furcht vor der trumpschen Zollkeule kündigte das Schweizer Unternehmen im vergangenen Frühjahr an, 23 Milliarden US-Dollar... ► Artikel lesen | |
| MERCK KGAA | 126,25 | 0,00 % | Big Blue IBM, Fastly & Merck KGaA - Was sagen Charts UND Fundamentaldaten wirklich? | ||
| GILEAD SCIENCES | 128,00 | -0,26 % | Gilead Sciences (GILD): Spannendes Chartbild - 150-USD-Trigger im Visier! | Vom HIV-Spezialisten zum Onkologie-Powerhouse! Rückblick Seit Anfang des Jahres befand sich die Aktie von Gilead Sciences in einem starken Aufwärtsimpuls, der sie von rund 122 US-Dollar bis auf ein... ► Artikel lesen | |
| AURORA CANNABIS | 3,175 | -0,47 % | Is Aurora Cannabis Stock Ready to 10X in the Next Pot Boom? | ||
| SANOFI | 81,31 | -0,26 % | Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung | DJ Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung
Von Colin Kellaher
DOW JONES--Der Ausschuss für Humanarzneimittel (CHMP) der Europäischen Arzneimittel-Agentur... ► Artikel lesen | |
| GSK | 24,930 | 0,00 % | GSK kauft kanadische 35Pharma für 950 Millionen US-Dollar | DJ GSK kauft kanadische 35Pharma für 950 Millionen US-Dollar
Von Sarah Sloat
DOW JONES--GSK hat die Übernahme des Biopharma-Unternehmens 35Pharma für 950 Millionen US-Dollar vereinbart. 35Pharma... ► Artikel lesen | |
| ABBVIE | 199,20 | -0,65 % | AbbVie Reports Positive Phase 3 Results For Risankizumab In Crohn's Disease | WASHINGTON (dpa-AFX) - AbbVie (ABBV) on Monday announced positive results from the Phase 3 AFFIRM study evaluating subcutaneous risankizumab in moderately to severely active Crohn's disease.... ► Artikel lesen | |
| ROCHE | 396,45 | -0,15 % | Hightech-Neubau für frühe Krankheitsdiagnosen: Drees & Sommer begleitete Roche bei Millionenprojekt | Penzberg (ots) - Im neu eingeweihten Diagnostik- und Innovationszentrum in Penzberg werden in Zukunft In-vitro-Diagnostika und spezifische Testverfahren entwickelt, um Krankheiten früh zu erkennen.Alzheimer... ► Artikel lesen | |
| CANOPY GROWTH | 0,930 | +0,11 % | Is It Time to Dump Your Shares of Canopy Growth?? | ||
| ELI LILLY | 866,30 | -0,48 % | Novo Nordisk setzt Eli Lilly unter Druck | Im frühen US-Handel zählen die Anteilscheine von Eli Lilly zu den schwächsten Titeln. Denn der dänische Rivale Novo Nordisk plant deutliche Preissenkungen für seine Blockbuster-Medikamente in den USA.... ► Artikel lesen | |
| MERCK & CO | 103,60 | -0,19 % | Leerink raises Merck stock price target on belzutifan growth | ||
| ASTRAZENECA | 173,20 | -0,66 % | UBS stuft ASTRAZENECA auf 'Buy' | ZÜRICH (dpa-AFX Analyser) - Die Schweizer Großbank UBS hat das Kursziel für Astrazeneca von 16300 auf 17600 Pence angehoben und die Einstufung auf "Buy" belassen. Astrazeneca stehe zum Ende dieses Jahrzehnts... ► Artikel lesen |
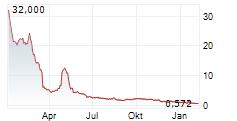
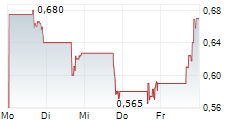
Das Unternehmen JAGUAR HEALTH INC kann der Branche Pharma zugeordnet werden. Der aktuelle Kurs liegt bei 0,805 Euro und damit 0,00 % im Plus.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu JAGUAR HEALTH INC finden Sie auf Wallstreet Online.