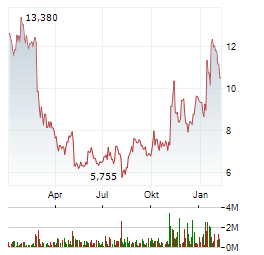
| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
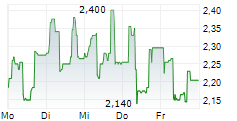
| 2,380 | 2,580 | 11.03. |
Chart-Typ:
Zeitraum:
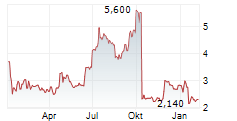
| Die Gründer von BioNTech kündigen ihren Abschied vom Unternehmen an und zusammen mit schwachen Zahlen lässt dies die Aktie abstürzen | Die meisten Anleger dürften bereits damit gerechnet haben, dass BioNTech keine überwältigenden Zahlen vorlegen würde. Schließlich mangelt es dem Unternehmen noch immer an Umsatzbringern, während die... ► Artikel lesen |
| BioNTech-Gründerausstieg drückt Kursziele - Kaufvoten bleiben | Der angekündigte Abgang von BioNTech-Gründern Ugur Sahin und Özlem Türeci hat bei der Analystengemeinde trotz eines als solide bewerteten Quartalsberichts gemischte Gefühle hervorgerufen. Zwei von drei... ► Artikel lesen |
| GOLDMAN SACHS stuft Biontech auf 'Buy' | NEW YORK (dpa-AFX Analyser) - Die US-Investmentbank Goldman Sachs hat das Kursziel für Biontech von 145 auf 132 US-Dollar gesenkt, aber die Einstufung auf "Buy" belassen. Der Ausstieg der Gründer habe... ► Artikel lesen |
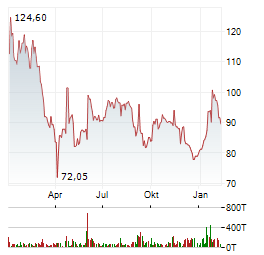
| PTA-NVR: QIAGEN N.V.: Veröffentlichung der Gesamtzahl der Stimmrechte nach § 41 WpHG | DJ PTA-NVR: QIAGEN N.V.: Veröffentlichung der Gesamtzahl der Stimmrechte nach § 41 WpHG
Gesamtstimmrechte gemäß -- 41 WpHG
QIAGEN N.V.: Veröffentlichung der Gesamtzahl der Stimmrechte nach... ► Artikel lesen |
| Shortseller-Positionen aktuell: Auto1, Energiekontor, freenet, Gerresheimer, Puma, Qiagen, TeamViewer | Wer Aktien leerverkauft, also auf fallende Kurse spekuliert (Shortselling), unterliegt bestimmten Transparenzpflichten. Aktuelle Meldungen über Leerverkäufe geben Aufschluss darüber. Diese Vorgaben... ► Artikel lesen |
| Shortseller-Positionen aktuell: freenet, Gerresheimer, Hypoport, Qiagen, Renk, Suss MicroTec, TeamViewer | Wer Aktien leerverkauft, also auf fallende Kurse spekuliert (Shortselling), unterliegt bestimmten Transparenzpflichten. Aktuelle Meldungen über Leerverkäufe geben Aufschluss darüber. Diese Vorgaben... ► Artikel lesen |
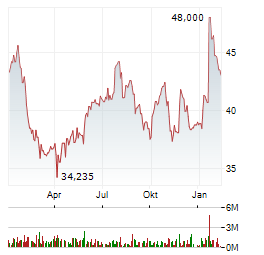
| Servier and Day One Biopharmaceuticals announce acquisition to expand Servier's rare oncology portfolio | Acquisition positions Servier as a leader in pediatric low-grade glioma and expands its pipeline with programs targeting adult and pediatric cancers with high unmet needs. Transaction represents... ► Artikel lesen |
| Day One Biopharmaceuticals, Inc.: Servier and Day One Biopharmaceuticals announce acquisition to expand Servier's rare oncology portfolio | Acquisition positions Servier as a leader in pediatric low-grade glioma and expands its pipeline with programs targeting adult and pediatric cancers with high unmet needs Transaction represents total... ► Artikel lesen |
| Day One Biopharmaceuticals, Inc.: Day One Reports Fourth Quarter and Full Year 2025 Financial Results and Reaffirms 2026 Outlook and Revenue Guidance | OJEMDA 2025 momentum reflected by Q4 and full year net product revenues of $52.8 million and $155.4 million, respectively 2026 U.S. net product revenue projected at $225 - $250 million Expanded pipeline... ► Artikel lesen |
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu XSPRAY PHARMA AB finden Sie auf Wallstreet Online.