| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
| 2,340 | 2,540 | 13:02 |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Do | XSpray Pharma AB: Xspray Pharma re-submits its FDA application for Dasynoc | 75 | GlobeNewswire (Europe) | Xspray Pharma AB (publ) has re-submitted its application for market approval for Dasynoc® to the U.S. Food and Drug Administration (FDA). Dasynoc® is the company's lead product candidate - an amorphous... ► Artikel lesen | |
| Do | XSpray Pharma AB: Xspray Pharma appoints Blake Leitch as CEO - Per Andersson continues as Chief Scientific Officer (CSO) | 169 | GlobeNewswire (Europe) | The Board of Directors of Xspray Pharma has today appointed Blake Leitch as CEO of Xspray Pharma, effective no later than June 1, 2026. He brings over 20 years of international experience of marketing... ► Artikel lesen | |
| 12.02. | XSpray Pharma AB: Interim Report Fourth Quarter 2025 | 58 | GlobeNewswire (Europe) | October-December 2025, GroupNet sales amounted to SEK 0 thousand (0)Earnings before tax amounted to SEK -37,009 thousand (-82,002)Earnings per share before dilution amounted to SEK -0.89 (-2.36)Cash... ► Artikel lesen | |
| 05.11.25 | XSpray Pharma AB: Interim Report Third Quarter 2025 | 199 | GlobeNewswire (Europe) | July-September 2025, GroupNet sales amounted to SEK 0 thousand (0)Earnings before tax amounted to SEK -47,332 thousand (-82,272)Earnings per share before dilution amounted to SEK -1.25 (-2.44)Cash flow... ► Artikel lesen | |
| 08.10.25 | XSpray Pharma AB: Xspray Pharma provides update on the FDA process for Dasynoc - observations at contract manufacturer delay approval | 252 | GlobeNewswire (Europe) | • FDA has issued a Complete Response Letter (CRL) regarding Xspray Pharma's New Drug Application (NDA) for Dasynoc, referring to GMP (Good Manufacturing Practice) observations at a contract manufacturer.... ► Artikel lesen | |
| 11.09.25 | XSpray Pharma AB: Xspray's rights issue oversubscribed - over-allotment issue increased and fully utilized | 283 | GlobeNewswire (Europe) | THIS PRESS RELEASE MAY NOT BE MADE PUBLIC, PUBLISHED OR DISTRIBUTED, DIRECTLY OR INDIRECTLY, IN OR INTO THE UNITED STATES OF AMERICA, AUSTRALIA, BELARUS, CANADA, HONG KONG, JAPAN, NEW ZEELAND, RUSSIA... ► Artikel lesen | |
| 26.08.25 | Listing of subscription rights and paid subscription shares of XSpray Pharma AB | 373 | GlobeNewswire | With effect from August 27, 2025, the subscription rights of XSpray Pharma AB will be traded on the list for Equity rights. Trading will continue up until and including September 04, 2025.
Instrument:
Subscription... ► Artikel lesen | |
| 25.08.25 | XSpray Pharma AB: Xspray publishes disclosure document regarding rights issue | 387 | GlobeNewswire (Europe) | THIS PRESS RELEASE MAY NOT BE MADE PUBLIC, PUBLISHED OR DISTRIBUTED, DIRECTLY OR INDIRECTLY, IN OR INTO THE UNITED STATES OF AMERICA, AUSTRALIA, BELARUS, CANADA, HONG KONG, JAPAN, NEW ZEELAND, RUSSIA... ► Artikel lesen | |
| XSPRAY PHARMA Aktie jetzt für 0€ handeln | |||||
| 21.08.25 | XFRA CAPITAL ADJUSTMENT INFORMATION - 21.08.2025 | 627 | Xetra Newsboard | Das Instrument 6O1 CA13810W1023 CANTER RESOURCES CORP. EQUITY wird cum Kapitalmassnahme gehandelt am 21.08.2025 und ex Kapitalmassnahme am 22.08.2025 The instrument 6O1 CA13810W1023 CANTER RESOURCES... ► Artikel lesen | |
| 19.08.25 | XSpray Pharma AB: Xspray Pharma Submits XS003 to the FDA - The Company's Second Product Candidate from the HyNap Platform | 506 | GlobeNewswire (Europe) | Xspray Pharma (Nasdaq Stockholm: XSPRAY) has submitted a New Drug Application (NDA) to the U.S. Food and Drug Administration (FDA) for its product candidate XS003 (nilotinib) for the treatment of chronic... ► Artikel lesen | |
| 15.08.25 | XSpray Pharma AB: Interim Report Second Quarter 2025 | 269 | GlobeNewswire (Europe) | April-June 2025, GroupNet sales amounted to SEK 0 thousand (0)Earnings before tax amounted to SEK -44,885 thousand (-53,620)Earnings per share before dilution amounted to SEK -2.35 (-1.64)Cash flow... ► Artikel lesen | |
| 12.08.25 | XSpray Pharma AB: Xspray Pharma signs license agreement with Handa Therapeutics - to receive up to double-digit royalty on Handa's net proceeds | 271 | GlobeNewswire (Europe) | Xspray Pharma ("Xspray") has entered into a license agreement with Handa Therapeutics ("Handa") granting Handa a non-exclusive license to certain Xspray patents. The license covers commercialization... ► Artikel lesen | |
| 11.07.25 | XSpray Pharma AB: XSpray Pharma achieves significant milestone - demonstrating bioequivalence with absorption advantages compared to Tasigna | 225 | GlobeNewswire (Europe) | Xspray Pharma has now completed the population pharmacokinetic (PopPK) modeling that constitutes key regulatory documentation ahead of submitting a New Drug Application (NDA) for the product candidate... ► Artikel lesen | |
| 27.06.25 | XSpray Pharma AB: Xspray Pharma Passes FDA Pre-Approval Inspection - Key Regulatory Milestone Achieved for Dasynoc | 533 | GlobeNewswire (Europe) | Xspray Pharma AB (publ) today announces that the U.S. Food and Drug Administration (FDA) has conducted a successful Pre-Approval Inspection (PAI) of the company's manufacturing lines, located at a contract... ► Artikel lesen | |
| 14.05.25 | XSpray Pharma AB: FDA sets PDUFA-date for Xspray Pharma's re-submitted application for Dasynoc | 406 | GlobeNewswire (Europe) | The U.S. Food and Drug Administration (FDA) has acknowledged receipt of Xspray Pharma's re-submitted NDA (New Drug Application) for Dasynoc®. The re-submission is based on a CRL (Complete Response Letter)... ► Artikel lesen | |
| 13.05.25 | XSpray Pharma AB: Bulletin from the annual general meeting of Xspray Pharma AB (publ) | 192 | GlobeNewswire (Europe) | The following resolutions were passed at the annual general meeting (the "AGM") of Xspray Pharma AB (publ) ("Xspray") on 13 May 2025.
Adoption of income statement and balance sheet for the financial... ► Artikel lesen | |
| 07.05.25 | XSpray Pharma AB: Interim Report First Quarter 2025 | 266 | GlobeNewswire (Europe) | January - March 2025, Group• Net sales amounted to SEK 0 thousand (0)• Earnings before tax amounted to SEK -42,321 thousand (-67,781)• Earnings per share before dilution amounted to SEK -1.14 (-2.17)•... ► Artikel lesen | |
| 08.04.25 | XSpray Pharma AB: Xspray Pharma re-submits its FDA application | 383 | GlobeNewswire (Europe) | Xspray Pharma AB (publ) has re-submitted its application for market approval for Dasynoc®, the company's lead product candidate, an amorphous dasatinib for the treatment of leukemia. The application... ► Artikel lesen |
18 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| QIAGEN | 41,960 | +1,38 % | Qiagen: Analysten sehen Wettbewerbssorgen als übertrieben | Nach Gesprächen mit dem Qiagen-Management auf Analysten-Konferenzen in den USA stufen Analysten die Aktie des Diagnostikspezialisten weiterhin positiv ein. Jefferies beließ es bei einer Kaufempfehlung... ► Artikel lesen | |
| STRUCTURE THERAPEUTICS | 62,98 | -2,63 % | Structure Therapeutics Inc.: Structure Therapeutics Reports Fourth Quarter and Full Year 2025 Financial Results and Recent Highlights | Positive results from the aleniglipron Phase 2 ACCESS programs in December 2025 demonstrated significant weight loss across all doses and up to 15.3% at 36 weeks Topline 44-week data from the ACCESS... ► Artikel lesen | |
| ARCELLX | 113,80 | -0,05 % | Aktien New York Ausblick: Leichte Verluste nach Trumps Zollankündigung | NEW YORK (dpa-AFX) - Die US-Börsen steuern am Montag vor dem Hintergrund neuer Zollunsicherheit auf einen etwas tieferen Handelsauftakt zu. Nachdem der Oberste Gerichtshof der USA dem Präsidenten Donald... ► Artikel lesen | |
| EVOTEC | 5,880 | +0,38 % | Evotec Aktie: Kann man "diesem Braten wirklich trauen"? | © Foto: Julia Koblitz auf Unsplash (Symbolbild)Neue Aufträge, ein Sparprogramm und frische Analystenkommentare. Die Evotec-Aktie sendet wieder Lebenszeichen. Doch wer die Geschichte dieses Hamburger... ► Artikel lesen | |
| BIONTECH | 93,10 | -0,21 % | Aufgrund von angeblichen Patentverletzungen zieht BioNTech gegen Moderna vor Gericht | Die Hersteller von Corona-Impfstoffen haben sich schon seit Jahren in den Haaren. Immer wieder gibt es Vorwürfe über Patentverletzungen. So auch in einem aktuellen Fall, bei dem BioNTech gegen den US-Hersteller... ► Artikel lesen | |
| VIR BIOTECHNOLOGY | 9,100 | -2,26 % | Vir Biotechnology +70%: Deal-Überraschung mit Ansage | Die Aktie von Vir Biotechnology ist vorbörslich um rund 70 Prozent nach oben geschossen. Auslöser der Kursexplosion ist vor allem ein milliardenschwerer Kooperationsdeal mit dem japanischen Pharmakonzern... ► Artikel lesen | |
| AVIDITY BIOSCIENCES | 14,750 | -79,74 % | Novartis Pharma AG: Novartis successfully completes acquisition of Avidity Biosciences, strengthening late-stage neuroscience pipeline and advancing xRNA strategy | Adds Avidity's differentiated muscle-directed Antibody Oligonucleotide Conjugates (AOC) platform and three late-stage programs to industry-leading neuromuscular pipelinePotentially unlocks multi-billion-dollar... ► Artikel lesen | |
| CARDIO DIAGNOSTICS | 6,490 | +24,33 % | Cardio Diagnostics Holdings, Inc. - 8-K, Current Report | ||
| PRAXIS PRECISION MEDICINES | 336,75 | -1,05 % | Praxis Precision Medicines, Inc.: Praxis Precision Medicines Provides Corporate Update and Reports Fourth Quarter and Full-Year 2025 Financial Results | Two new drug applications (NDA) for ulixacaltamide in essential tremor (ET) and for relutrigine in SCN2A and SCN8A developmental and epileptic encephalopathies (DEEs) have been submitted to the U.S.... ► Artikel lesen | |
| VERA THERAPEUTICS | 40,820 | -3,48 % | Vera Therapeutics, Inc. - 10-K, Annual Report | ||
| RELAY THERAPEUTICS | 10,250 | +12,02 % | Relay Therapeutics, Inc. - S-8, Securities to be offered to employees in employee benefit plans | ||
| ADMA BIOLOGICS | 15,580 | +2,70 % | ADMA Q4 EPS Up 14% Y/Y, Revenues Gain From Strong Asceniv Performance | ||
| EDGEWISE THERAPEUTICS | 30,450 | +2,77 % | Edgewise Therapeutics Reports Fourth Quarter and Full Year 2025 Financial Results with Strong Progress Across Muscular Dystrophy and Cardiovascular Programs | - CIRRUS-HCM 12-week data of EDG-7500 in obstructive and nonobstructive hypertrophic cardiomyopathy (HCM) expected in H1 2026 -
- Phase 1 healthy adult trial... ► Artikel lesen | |
| COGENT BIOSCIENCES | 38,870 | -1,55 % | Analyst Bullish on Cogent (COGT) Amid Expected Drug Approval | ||
| PRIME MEDICINE | 4,630 | +5,71 % | Prime Medicine, Inc.: Prime Medicine Reports Third Quarter 2025 Financial Results and Provides Business Updates | -- New preclinical data for PM577 in Wilson's Disease (WD) to be presented at AASLD; on-track to file IND and/or CTA in H1'26, with initial clinical data expected in 2027 -- -- Nominated PM647 as... ► Artikel lesen |
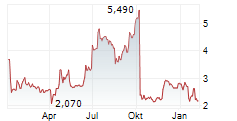
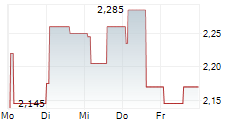
XSPRAY PHARMA AB gehört der Branche Biotechnologie an. Mit einer aktuellen Kursveränderung von +0,115 (+5,10 %) liegt der Kurs bei 2,370 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu XSPRAY PHARMA AB finden Sie auf Wallstreet Online.