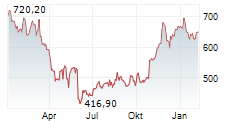
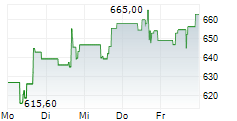
WASHINGTON (dpa-AFX) - Regeneron Pharmaceuticals, Inc. (REGN) announced that the European Commission has granted conditional marketing approval of Lynozyfic, or linvoseltamab, to treat adults with relapsed and refractory multiple myeloma. The indication is specific to those who have received at least three prior therapies, including a proteasome inhibitor, an immunomodulatory agent and an anti-CD38 monoclonal antibody, and have demonstrated disease progression on the last therapy.
The company noted that, in the U.S., the FDA accepted for review the Biologics License Application for linvoseltamab in adults with R/R MM with a target action date of July 10, 2025.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News