WASHINGTON (dpa-AFX) - Regeneron Pharmaceuticals, Inc. (REGN) announced the presentation of 27 abstracts, including eight oral sessions, featuring EYLEA HD (aflibercept) Injection 8 mg at the upcoming ARVO 2025 Annual Meeting, taking place from May 4-8 in Salt Lake City.
The findings cover its use in wet age-related macular degeneration (wAMD), diabetic macular edema (DME), and diabetic retinopathy (DR), offering insights into real-world applications that reinforce pivotal trial outcomes.
Boaz Hirshberg, M.D., Senior Vice President of Clinical Development, stated that the data demonstrate a growing body of evidence supporting EYLEA HD as the new standard of care for wAMD, DME, and DR. He highlighted that nearly 40,000 patients have shown improved vision and longer dosing intervals in clinical practice, emphasizing the positive real-world impact of EYLEA HD for serious retinal diseases.
Among the key presentations are four analyses evaluating real-world use of EYLEA HD in treatment-naïve and previously treated wAMD and DME patients, a network meta-analysis comparing EYLEA HD and faricimab efficacy and injection frequency, and a modeling study assessing the economic benefits of EYLEA HD versus faricimab over three years. Common adverse reactions (?3%) with EYLEA HD include cataract, conjunctival hemorrhage, increased intraocular pressure, ocular discomfort, blurred vision, vitreous floaters, vitreous detachment, corneal epithelium defects, and retinal hemorrhage.
At ARVO, notable presentations include early real-world insights into EYLEA HD use in treatment-naïve and switching DME patients by Nitish Mehta, M.D. and Michael Javaheri, M.D., respectively, on May 5. Economic modeling data comparing aflibercept 8 mg and faricimab will be presented by Andreas Kuznik. Additional sessions on May 6 will showcase real-world use in wAMD patients, both treatment-naïve and switchers, led by Ferhina Ali, M.D. and Theodore Leng, M.D.
Further, analyses of injection numbers comparing EYLEA HD and faricimab by Steven Sherman, volumetric fluid assessment studies from the CANDELA trial by John Mamone, M.D., and 156-week data from the PULSAR trial by Timothy Lai, M.D., will be presented. Other highlights include early results from the global SPECTRUM study, 96-week pooled analyses from the CANDELA, PHOTON, and PULSAR trials, and insights into socioeconomic factors influencing bevacizumab use in DME patients.
The EYLEA HD clinical trial program, including PULSAR and PHOTON, involved double-masked, active-controlled pivotal studies where patients were assigned to receive EYLEA HD every three or four months, or standard EYLEA every two months. In year one, dosing intervals for EYLEA HD could be shortened for disease progression but not extended until the second year. An optional extension phase allowed adjustments between two to six months based on disease activity. The CANDELA Phase 2 trial further evaluated extended dosing regimens for EYLEA HD compared to standard EYLEA in wAMD patients.
wAMD is characterized by abnormal blood vessel growth under the macula, leading to fluid leakage and potential vision loss, affecting approximately 1.4 million Americans. Diabetic retinopathy (DR) stems from retinal microvascular damage due to poor blood sugar control and can progress from nonproliferative (NPDR) to proliferative (PDR) stages, risking severe vision impairment. Diabetic macular edema (DME) occurs at any DR stage, with about 1.5 million adults diagnosed in the U.S., and six million having DR without DME.
EYLEA HD, a high-dose version of Regeneron's established EYLEA treatment, offers comparable efficacy and safety with fewer injections, based on 16 pivotal trials. It is approved for wAMD, DME, and DR treatment and marketed as EyleaT 8 mg in the European Union and Japan. Regeneron holds exclusive U.S. rights, while Bayer manages ex-U.S. markets, sharing profits equally.
Regeneron's ophthalmology development program is grounded in angiogenesis research, with ongoing efforts to address geographic atrophy, glaucoma, and inherited retinal diseases.
Important Safety Information: EYLEA HD and EYLEA are administered via intravitreal injection. Patients should not use EYLEA HD if they have ocular or periocular infections, active intraocular inflammation, or known hypersensitivity to aflibercept or any excipients. Patients are advised to report symptoms such as eye pain, redness, or vision changes immediately following injection.
Indications: EYLEA HD is approved for treating wAMD, DME, and DR. EYLEA 2 mg is approved for wAMD, macular edema following RVO, DME, DR, and retinopathy of prematurity (ROP).
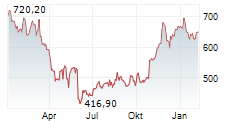
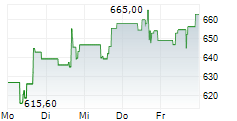
Monday, REGN closed at $610.86, up 1.36%, and is trading at $614 after hours, gaining an additional 0.51% on the Nasdaq Global Select Market.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News