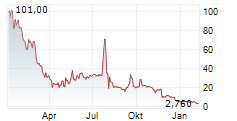
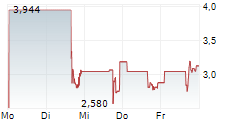
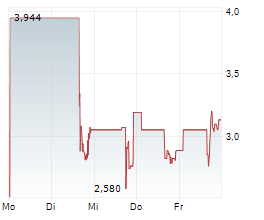
New York, New York--(Newsfile Corp. - May 1, 2025) - Psyence Biomedical Ltd. (NASDAQ: PBM) ("Psyence BioMed" or the "Company") today announced the effective date of its 1-for-7.97 share consolidation (reverse stock split) of the Company's issued and outstanding common shares. At a Special Meeting of Stockholders held on April 16, 2025, shareholders approved a share consolidation at a ratio of up to 1-for-50. Following this approval, the Company's Board of Directors authorized a 1-for-7.97 consolidation ratio, which will be implemented as the final share structure.
The Company's common shares are expected to begin trading on a post-consolidated basis at the opening of the market on Monday, May 5, 2025. Following the consolidation, the Company's common shares will continue to trade under the symbol "PBM" on the Nasdaq Capital Market, with a new CUSIP number 74449F308.
The consolidation is part of the Company's plan to regain compliance with the minimum bid price requirement of Nasdaq Listing Rule 5450(a)(1) (the "Nasdaq Rule 5450") for continued listing on The Nasdaq Capital Market.
At the effective time of the consolidation, every 7.97 issued and outstanding shares of the Company will automatically be combined into one issued and outstanding common share. The number of shares and the exercise price of the Company's outstanding warrants and other equity instruments will also be adjusted proportionately in accordance with their respective terms. As of May 1, 2025, the Company had 4,648,610 common shares issued and outstanding. As a result of the consolidation, the Company will have 583,263 common shares issued and outstanding.
No fractional shares will be issued in connection with the consolidation. Any fractional share resulting from the consolidation will be rounded down to the nearest whole share if the fraction is less than one-half of a share and rounded up to the nearest whole share if the fraction is at least one-half of a share. The consolidation affects all shareholders uniformly and will not alter any shareholder's percentage interest in the Company, except for minor adjustments resulting from the treatment of fractional shares. The share consolidation occurs at the registered shareholder level. Shareholders who hold their common shares through brokers, banks, or other nominees (i.e., in 'street name') are considered beneficial holders and may experience a delay in the reflection of the consolidation in their accounts, depending on the procedures of their broker, bank, or nominee.
Continental Stock Transfer & Trust Company is acting as the exchange agent and transfer agent for the consolidation. Shareholders holding their shares in book-entry form or through brokerage accounts are not required to take any action. Beneficial holders are encouraged to contact their broker, bank, or custodian with any questions regarding the effect of the share consolidation.
About Psyence BioMed:
Psyence BioMed aims to be one of the few multi-asset, vertically integrated biopharmaceutical companies specializing in psychedelic-based therapeutics. It is the first life sciences biotechnology company focused on developing nature-derived (non-synthetic) psilocybin-based psychedelic medicine to be listed on Nasdaq. Psyence is dedicated to addressing unmet mental health needs, particularly in palliative care. The name 'Psyence' merges 'psychedelics' and 'science,' reflecting the company's commitment to an evidence-based approach in developing safe, effective, and FDA-approved nature-derived psychedelic treatments for a broad range of mental health disorders.
Learn more at www.psyencebiomed.com and on LinkedIn.
Contact Information for Psyence Biomedical Ltd.
Email: ir@psyencebiomed.com
Media Inquiries: media@psyencebiomed.com
General Information: info@psyencebiomed.com
Phone: +1 416-477-1708
Investor Contact:
Michael Kydd
Investor Relations Advisor
michael@psyencebiomed.com
Forward-Looking Statements
This communication contains "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. Such statements may relate to future financial and operating results, plans, objectives, expectations, and intentions regarding future operations, products, services, and other matters. Words such as "expects," "will," "intends," "believes," "plans," "anticipates," "projects," "targets," and similar expressions are intended to identify forward-looking statements.
Forward-looking statements in this communication include statements regarding the timing and implementation of the share consolidation, the continued listing of the Company's securities on Nasdaq, and the anticipated impact of the consolidation. These statements are based on current assumptions and expectations, including that the share consolidation will be completed without delay, and that the Company will continue to meet Nasdaq's ongoing listing standards.
There are numerous risks and uncertainties that may cause actual results or performance to differ materially from those expressed or implied in these forward-looking statements, including, but not limited to: (i) delays or challenges in completing the share consolidation; (ii) the Company's ability to maintain compliance with Nasdaq's continued listing standards; (iii) potential volatility in the Company's share price following the consolidation; (iv) changes in the regulatory, competitive, and economic landscape; and (v) risks associated with the Company's development plans and clinical trials.
These and other important risks and uncertainties are described in the "Risk Factors" section of the Company's final prospectus (File No. 333-284444), filed with the U.S. Securities and Exchange Commission on January 24, 2025, and in other documents filed by the Company from time to time with the SEC. Actual results and future events could differ materially from those anticipated in such statements. Readers are cautioned not to place undue reliance on forward-looking statements, which speak only as of the date they are made. Except as required by law, Psyence Biomed does not undertake any obligation to update or revise forward-looking statements.
The Company does not make any medical, treatment, or health benefit claims regarding its proposed products. The U.S. Food and Drug Administration, Health Canada, or similar regulatory bodies have not evaluated claims regarding psilocybin, psilocybin analogues, or other psychedelic or nutraceutical products. The efficacy of such products has not been confirmed by authorized clinical research. There is no assurance that the use of such compounds can diagnose, treat, cure, or prevent any disease or condition. Clinical trials and regulatory approvals are required and, if not obtained, may have a material adverse impact on the Company's business.

To view the source version of this press release, please visit https://www.newsfilecorp.com/release/250415
SOURCE: Psyence Biomed