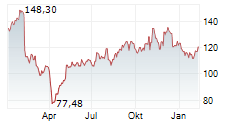
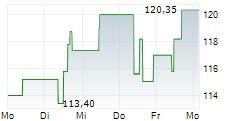
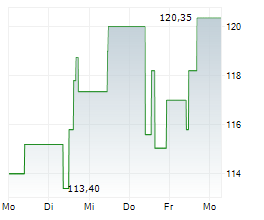
WASHINGTON (dpa-AFX) - Neurocrine Biosciences, Inc. (NBIX) Thursday announced positive results from the Phase 3 CAHtalyst Pediatric study of CRENESSITY in Congenital adrenal hyperplasia, a group of inherited disorders of the adrenal gland.
The CAHtalyst Pediatric study tested two questions- one, whether four weeks of treatment with CRENESSITY could improve androgen control, and second, if an additional 24 weeks of CRENESSITY treatment could enable customized glucocorticoid (GC) down-titration while androstenedione levels were maintained or improved.
The analyses of the all subgroups showed that pediatric patients with classic congenital adrenal hyperplasia maintained or improved their androstenedione levels with CRENESSITY while reducing glucocorticoid dosing. Additionally, CRENESSITY significantly reduced adrenal androgens at Week 4.
Overproduction of androstenedione, a key adrenal androgen, in pediatric patients with congenital adrenal hyperplasia can lead to abnormal growth and development, premature puberty and various developmental challenges.
These data will be presented at the 2025 Joint Congress of European Society for Paediatric Endocrinology and the European Society of Endocrinology in Copenhagen, Denmark.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News