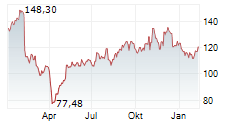
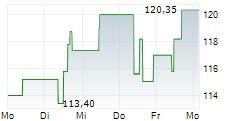
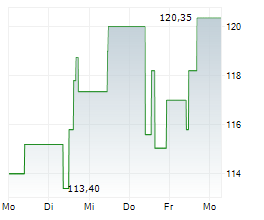
WASHINGTON (dpa-AFX) - Neurocrine Biosciences, Inc. (NBIX) Friday announced new encouraging data from a Phase 4 study of Ingrezza in patients with tardive dyskinesia, a neurological disorder characterized by involuntary movements.
The analyses were conducted using data from patients who participated in a Phase 4 withdrawal study. Patients in the study received Ingrezza for eight weeks, after which they were randomized to either continue Ingrezza or receive placebo for an additional eight weeks.
New data from the study showed that patients with tardive dyskinesia who received continued treatment with Ingrezza demonstrated improvements across functional and health-related quality of life (HRQoL) measures.
Patients who received Ingrezza for eight weeks experienced significant improvements in multiple areas of HRQoL, including mobility, self-care, usual activities and pain/discomfort. Those who received Ingrezza for an additional eight weeks saw continued improvements in all HRQoL dimensions, including significant improvements in mobility and anxiety/depression compared with those who were on placebo.
The analyses were presented at the 2025 International Society for Pharmacoeconomics and Outcomes Research (ISPOR) Conference in Montreal, Canada.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News