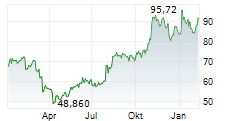
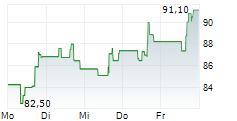
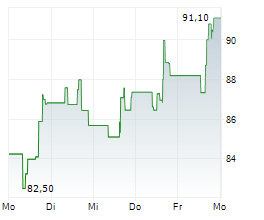
WASHINGTON (dpa-AFX) - Incyte Corp. (INCY) announced that primary results from the Phase 3 POD1UM-303/InterAACT 2 trial of retifanlimab (Zynyz)-a humanized monoclonal antibody targeting PD-1-combined with carboplatin and paclitaxel, have been published in The Lancet. The study focused on adults with inoperable, locally recurrent, or metastatic squamous cell carcinoma of the anal canal (SCAC) who had not previously received systemic chemotherapy.
According to the company, the POD1UM-303/InterAACT2 trial results showed that the study met its primary endpoint by demonstrating a statistically significant improvement in progression-free survival (PFS) in patients with inoperable locally recurrent or metastatic SCAC not previously treated with systemic chemotherapy, as assessed by blinded independent central review (BICR) using RECIST v1.1.2 Adding retifanlimab to carboplatin and paclitaxel resulted in a clinically meaningful 37% reduction in the risk of progression or death. Patients in the retifanlimab and chemotherapy combination group achieved a median PFS of 9.3 months compared to 7.4 months for patients in the placebo combination group.
In May 2025, the FDA approved Zynyz (retifanlimab-dlwr) in combination with carboplatin and paclitaxel (platinum-based chemotherapy) for the first-line treatment of adult patients with inoperable locally recurrent or metastatic SCAC. In addition, the FDA granted approval for Zynyz as a single agent for the treatment of adult patients with locally recurrent or with metastatic SCAC with disease progression on or intolerance to platinum-based chemotherapy.
Incyte has also submitted a Type II variation Marketing Authorization Application (MAA) to the European Medicines Agency (EMA) for retifanlimab in advanced SCAC and in March 2025 submitted and received acceptance of a Japanese New Drug Application (J-NDA) by the Pharmaceuticals and Medical Devices Agency (PMDA) for retifanlimab in advanced SCAC.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News