Funding secured
First quarter, 1 May - 31 July 2025
- Net sales amounted to SEK 811k (1,675k).
- Operating result (EBIT) amounted to SEK -6,552 (-7,620k).
- Net result for the period amounted to SEK -6,619k (-7,529k).
- Earnings per share before dilution amounted to SEK -1.64 (-1.87). Earnings per share after dilution amounted to SEK -1.64 (-1.87).
- Cash flow from operating activities amounted to SEK 1,926k (2,480k).
- Net cash flow amounted to SEK 774k (-209k).
- Second payment of just over SEK 5 million was made for the EU-funded Accelerator project supporting the development of Qlucore Diagnostics and Qlucore Insights for bladder cancer and adult acute myeloid leukemia (AML).
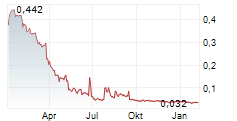
- After the reporting period. A rights issue has resulted in a liquidity injection of approximately SEK 14.5 million net in September. The dilution effect amounted to approximately 89 percent. The three largest shareholders are now Palmstierna Invest, Mikrolund and Daniel Nilsson, who together hold 51.5 percent of the shares.
CEO's statement
During the period, focus has been on securing financing. Intensive discussions are underway, both with existing owners as well as with new investors to ensure financing. At the end of July, the new share issue, with a net contribution of approximately SEK 14.75 million, was 100% guaranteed. The planned liquidity injection will ensure stable operations. The plan consists mainly of three parts. First, continued work on developing new diagnostic tests within the framework of the EU-funded accelerator project. Second, increased market presence by establishing cooperation with more agents and distributors. Third, implementation of a cost saving program of more than 25%.
The work on cost savings, primarily through personnel reductions, was initiated in June and the effects are expected to take full effect in November.
Net sales during the first quarter amounted to SEK 811k (1,675k), which is a decrease of 52 percent. This is primarily due to a relatively large customer in drug development having made cost cuts, reorganized their operations and having chosen not to renew their licenses. We now also report on the ARR measure for Qlucore Omics Explorer (annual recurring revenue) to provide a better accrual of revenue from multi-year deals. As of last July, ARR was SEK 10,019k (11,783k), compared to the previous quarter of SEK 10,681k.
Diagnostics
Sales and marketing are actively underway with both the leukemia model and the lung cancer model. We have decided to supplement our own sales organization with distributors and agents and the work of identifying and securing contracts is ongoing.
Our product is unique and there are no comparable IVDR approved products on the market. CE marking according to the IVDR regulations for medical devices is a requirement for use in diagnostics within healthcare in Europe.
We are at the forefront of using genetic information to classify patients with regulatory approved products. Now we have a stable regulatory-approved platform to broaden our product portfolio. The degree of reuse, of both documentation and code invested in Qlucore Diagnostics, is high for future diagnostic models. This means that we can work in parallel with the projects within the Accelerator project; bladder cancer and acute myeloid leukemia in adults (AML).
These cancers represent strategically important therapy areas with a large medical need for improved diagnostics. Together, they constitute approximately 16% of all cancer cases. In figures, this amounts to approximately 1 million cases per year in Europe and the USA, increasing to just under 2.5 million when China, India and parts of South America are included.
Distributors and agents
In July, the agreement with JingQiang, our new agent in China, was finalized. They will sell both Qlucore Omics Explorer and Qlucore Insights.
After the reporting period
In early August, we received the first order for Qlucore Insights with the lung model. The customer, Mubadala Health Dubai, ordered both the leukemia and lung cancer models, demonstrating the scalability of the solution.
Global landscape
It is difficult to assess how the geopolitical situation and the weaker global economy have affected Qlucore's operations during the period. We are experiencing restrictive purchasing behaviors. When possible, customers are also postponing renewal for a couple of months. There is a clear risk that cuts in American research funding will negatively affect us.
___________________
It is very satisfying that we have both secured funding and welcomed our first customer for the lung model in Qlucore Insight's, an important milestone for the company.
Carl-Johan Ivarsson, CEO
Certified Advisor
FNCA Sweden AB
Web: www.fnca.se
Contacts
Press contact:
Maria Falck Miniotis, Marketing & Communications Manager
Phone: +46 (0) 736 10 26 65
Email: maria.falck@qlucore.com
Carl-Johan Ivarsson, CEO
Phone: +46 (0) 46 286 31 10
Email: carl-johan.ivarsson@qlucore.com
About Qlucore
Qlucore is a leading provider of new generation intuitive bioinformatics software for research and precision and companion diagnostics. Qlucore's mission is to make it easier to analyze the huge amounts of complex data generated by innovations in the fields of genomics and proteomics by providing powerful visualization-based bioinformatics data analysis tools for research and precision diagnostics. Qlucore Omics Explorer software is a easy to use bioinformatics software for research in the life science, plant- and biotech industries, as well as in academia. Qlucore Diagnostics and Qlucore Insights are software platforms with built in AI-based machine learning for multi-omics companion and precision diagnostics. Qlucore was founded in 2007 in Lund, Sweden and has customers in about 20 countries around the world, with sales offices in Europe and North America, and distribution in several countries in Asia. Qlucore is listed on the Nasdaq First North Growth market. www.qlucore.com
This information is information that Qlucore is obliged to make public pursuant to the EU Market Abuse Regulation. The information was submitted for publication, through the agency of the contact persons set out above, at 2025-09-24 18:30 CEST.