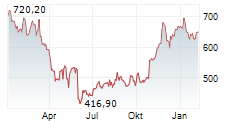
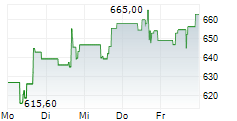
WASHINGTON (dpa-AFX) - Regeneron Pharmaceuticals (REGN) announced the FDA has approved Evkeeza ANGPTL3 antibody as an adjunct to diet and exercise and other lipid-lowering therapies for the treatment of children from age 1 to less than 5 years old with homozygous familial hypercholesterolemia.
Evkeeza was approved in 2021 for adults and adolescents aged 12 years and older with HoFH based on a placebo-controlled trial showing Evkeeza, when added to standard lipid-lowering therapies, could lower LDL-C by about 50% compared to placebo. It was approved for children aged 5 to 11 in 2023.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News