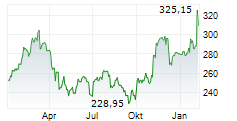
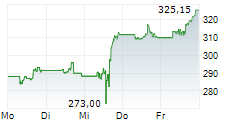
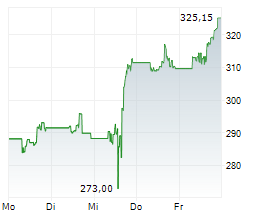
THOUSAND OAKS (dpa-AFX) - Amgen Inc. (AMGN) on Thursday announced positive results form the Phase 3 VESALIUS-CV study of Repatha in adults with high cardiovascular risk without prior heart attack or stroke.
The study met its dual primary endpoints demonstrating that Repatha significantly reduced the risk of major adverse cardiovascular events (MACE) in individuals without a prior history of heart attack or stroke.
The VESALIUS-CV primary endpoints were time to first occurrence of a composite of coronary heart disease (CHD) death, heart attack or ischemic stroke as well as time to first occurrence of a composite of CHD death, heart attack, ischemic stroke or any ischemia-driven arterial revascularization. The results were both statistically and clinically significant.
Repatha was first approved in 2015 to treat certain patients with high cholesterol.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News