WASHINGTON (dpa-AFX) - Regeneron Pharmaceuticals Inc. (REGN) announced that the U.S. Food and Drug Administration has approved its PD-1 inhibitor, Libtayo (cemiplimab-rwlc), as an adjuvant treatment for adult patients with cutaneous squamous cell carcinoma (CSCC) who are at high risk of recurrence following surgery and radiation therapy.
In addition to the U.S. approval, a regulatory application for Libtayo is currently under review in the European Union, with a decision anticipated in the first half of 2026.
Cutaneous squamous cell carcinoma is among the most common forms of skin cancer globally, with approximately 1.8 million cases diagnosed annually in the United States alone. While many cases are effectively treated with surgery and radiation, a significant number of patients remain at risk for advanced disease recurrence. Samantha R. Guild, President of AIM at the Skin Cancer Foundation, emphasized the seriousness of these risks and the importance of new treatment options.
Regeneron also clarified that the FDA-approved supplemental Biologics License Application (sBLA) for Libtayo does not include Catalent Indiana, LLC as a filling site.
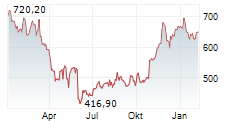
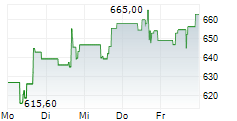
REGN closed Wednesday's regular trading at $563.86 down $19.38 or 3.32%. But in the after-hours trading, the stock gained $1.14 or 0.20%.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News